[English] 日本語
 Yorodumi
Yorodumi- PDB-8pn2: CryoEM structure of Nal1 protein, allele IR64, from Oryza sativa ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8pn2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of Nal1 protein, allele IR64, from Oryza sativa indica cultivar | |||||||||
 Components Components | Protein NARROW LEAF 1 | |||||||||
 Keywords Keywords |  PLANT PROTEIN / PLANT PROTEIN /  Serine protease Serine protease | |||||||||
| Function / homology | stem vascular tissue pattern formation / internode patterning / regulation of leaf development / leaf vascular tissue pattern formation / Peptidase S1, PA clan /  nucleoplasm / nucleoplasm /  cytoplasm / ADENOSINE-5'-TRIPHOSPHATE / Protein NARROW LEAF 1 cytoplasm / ADENOSINE-5'-TRIPHOSPHATE / Protein NARROW LEAF 1 Function and homology information Function and homology information | |||||||||
| Biological species |   Oryza sativa Indica Group (long-grained rice) Oryza sativa Indica Group (long-grained rice) | |||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.63 Å cryo EM / Resolution: 2.63 Å | |||||||||
 Authors Authors | Huang, L.Y. / Rety, S. / Xi, X.G. | |||||||||
| Funding support |  China, China,  France, 2items France, 2items
| |||||||||
 Citation Citation |  Journal: Nat Plants / Year: 2024 Journal: Nat Plants / Year: 2024Title: The catalytic triad of rice NARROW LEAF1 involves H234. Authors: Ling-Yun Huang / Na-Nv Liu / Wei-Fei Chen / Xia Ai / Hai-Hong Li / Ze-Lin Zhang / Xi-Miao Hou / Philippe Fossé / Olivier Mauffret / Dong-Sheng Lei / Stephane Rety / Xu-Guang Xi /   Abstract: NARROW LEAF1 (NAL1) exerts a multifaceted influence on leaf morphology and crop yield. Recent crystal study proposed that histidine 233 (H233) is part of the catalytic triad. Here we report that ...NARROW LEAF1 (NAL1) exerts a multifaceted influence on leaf morphology and crop yield. Recent crystal study proposed that histidine 233 (H233) is part of the catalytic triad. Here we report that unlike suggested previously, H234 instead of H233 is a component of the catalytic triad alongside residues D291 and S385 in NAL1. Remarkably, residue 233 unexpectedly plays a pivotal role in regulating NAL1's proteolytic activity. These findings establish a strong foundation for utilizing NAL1 in breeding programs aimed at improving crop yield. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8pn2.cif.gz 8pn2.cif.gz | 467.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8pn2.ent.gz pdb8pn2.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  8pn2.json.gz 8pn2.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/pn/8pn2 https://data.pdbj.org/pub/pdb/validation_reports/pn/8pn2 ftp://data.pdbj.org/pub/pdb/validation_reports/pn/8pn2 ftp://data.pdbj.org/pub/pdb/validation_reports/pn/8pn2 | HTTPS FTP |
|---|
-Related structure data
| Related structure data | 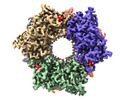 17768MC  8pmeC  8pmgC  8pmiC  8pmlC  8pmmC  8pn1C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 63822.469 Da / Num. of mol.: 6 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Oryza sativa Indica Group (long-grained rice) Oryza sativa Indica Group (long-grained rice)Gene: NAL1, GFP, LSCHL4, SPIKE, Os04g0615000, LOC_Os04g52479, OsJ_16147 Plasmid: pET15b-sumo / Production host:   Escherichia coli K-12 (bacteria) / References: UniProt: B4XT64 Escherichia coli K-12 (bacteria) / References: UniProt: B4XT64#2: Chemical | ChemComp-ATP /  Adenosine triphosphate Adenosine triphosphate#3: Chemical | ChemComp-MG / Has ligand of interest | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Nal1 / Type: COMPLEX / Entity ID: #1 / Source: RECOMBINANT |
|---|---|
| Molecular weight | Experimental value: NO |
| Source (natural) | Organism:   Oryza sativa Japonica Group (Japanese rice) Oryza sativa Japonica Group (Japanese rice) |
| Source (recombinant) | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Buffer solution | pH: 7.5 / Details: 20mM Tris-HCl 100mM NaCl |
| Specimen | Conc.: 1 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 282 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 50000 nm / Nominal defocus min: 5000 nm / Cs Bright-field microscopy / Nominal defocus max: 50000 nm / Nominal defocus min: 5000 nm / Cs : 2.7 mm / Alignment procedure: BASIC : 2.7 mm / Alignment procedure: BASIC |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Electron dose: 49.02 e/Å2 / Detector mode: SUPER-RESOLUTION / Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Num. of real images: 700 |
| Image scans | Movie frames/image: 32 |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 216590 | ||||||||||||||||||||||||||||
| Symmetry | Point symmetry : D3 (2x3 fold dihedral : D3 (2x3 fold dihedral ) ) | ||||||||||||||||||||||||||||
3D reconstruction | Resolution: 2.63 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 203380 / Algorithm: FOURIER SPACE / Num. of class averages: 1 / Symmetry type: POINT | ||||||||||||||||||||||||||||
| Atomic model building | Protocol: FLEXIBLE FIT / Space: REAL | ||||||||||||||||||||||||||||
| Atomic model building | PDB-ID: 8PME Accession code: 8PME / Source name: PDB / Type: experimental model | ||||||||||||||||||||||||||||
| Refinement | Cross valid method: NONE Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2 | ||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 39.58 Å2 | ||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller



 PDBj
PDBj