[English] 日本語
 Yorodumi
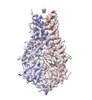
Yorodumi- PDB-8eqt: Structure of SARS-CoV-2 Orf3a in plasma membrane-like environment... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8eqt | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of SARS-CoV-2 Orf3a in plasma membrane-like environment, MSP1D1 nanodisc | ||||||
 Components Components | ORF3a protein | ||||||
 Keywords Keywords |  VIRAL PROTEIN / VIRAL PROTEIN /  Membrane protein / Membrane protein /  SARS-CoV-2 SARS-CoV-2 | ||||||
| Function / homology |  Function and homology information Function and homology informationhost cell lysosome / induction by virus of host reticulophagy / Maturation of protein 3a / SARS-CoV-2 modulates autophagy / inorganic cation transmembrane transport / host cell endoplasmic reticulum /  voltage-gated calcium channel complex / SARS-CoV-2 targets host intracellular signalling and regulatory pathways / voltage-gated calcium channel complex / SARS-CoV-2 targets host intracellular signalling and regulatory pathways /  voltage-gated potassium channel complex / molecular function activator activity ...host cell lysosome / induction by virus of host reticulophagy / Maturation of protein 3a / SARS-CoV-2 modulates autophagy / inorganic cation transmembrane transport / host cell endoplasmic reticulum / voltage-gated potassium channel complex / molecular function activator activity ...host cell lysosome / induction by virus of host reticulophagy / Maturation of protein 3a / SARS-CoV-2 modulates autophagy / inorganic cation transmembrane transport / host cell endoplasmic reticulum /  voltage-gated calcium channel complex / SARS-CoV-2 targets host intracellular signalling and regulatory pathways / voltage-gated calcium channel complex / SARS-CoV-2 targets host intracellular signalling and regulatory pathways /  voltage-gated potassium channel complex / molecular function activator activity / : / protein complex oligomerization / monoatomic ion channel activity / host cell endosome / Translation of Structural Proteins / Virion Assembly and Release / Induction of Cell-Cell Fusion / Attachment and Entry / host cell endoplasmic reticulum membrane / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / extracellular region / identical protein binding / voltage-gated potassium channel complex / molecular function activator activity / : / protein complex oligomerization / monoatomic ion channel activity / host cell endosome / Translation of Structural Proteins / Virion Assembly and Release / Induction of Cell-Cell Fusion / Attachment and Entry / host cell endoplasmic reticulum membrane / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / extracellular region / identical protein binding /  plasma membrane plasma membraneSimilarity search - Function | ||||||
| Biological species |   Severe acute respiratory syndrome coronavirus 2 Severe acute respiratory syndrome coronavirus 2 | ||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.4 Å cryo EM / Resolution: 3.4 Å | ||||||
 Authors Authors | Miller, A.N. / Houlihan, P.R. / Matamala, E. / Cabezas-Bratesco, D. / Lee, G.Y. / Cristofori-Armstrong, B. / Dilan, T.L. / Sanchez-Martinez, S. / Matthies, D. / Yan, R. ...Miller, A.N. / Houlihan, P.R. / Matamala, E. / Cabezas-Bratesco, D. / Lee, G.Y. / Cristofori-Armstrong, B. / Dilan, T.L. / Sanchez-Martinez, S. / Matthies, D. / Yan, R. / Yu, Z. / Ren, D. / Brauchi, S.E. / Clapham, D.E. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Elife / Year: 2023 Journal: Elife / Year: 2023Title: The SARS-CoV-2 accessory protein Orf3a is not an ion channel, but does interact with trafficking proteins. Authors: Alexandria N Miller / Patrick R Houlihan / Ella Matamala / Deny Cabezas-Bratesco / Gi Young Lee / Ben Cristofori-Armstrong / Tanya L Dilan / Silvia Sanchez-Martinez / Doreen Matthies / Rui ...Authors: Alexandria N Miller / Patrick R Houlihan / Ella Matamala / Deny Cabezas-Bratesco / Gi Young Lee / Ben Cristofori-Armstrong / Tanya L Dilan / Silvia Sanchez-Martinez / Doreen Matthies / Rui Yan / Zhiheng Yu / Dejian Ren / Sebastian E Brauchi / David E Clapham /   Abstract: The severe acute respiratory syndrome associated coronavirus 2 (SARS-CoV-2) and SARS-CoV-1 accessory protein Orf3a colocalizes with markers of the plasma membrane, endocytic pathway, and Golgi ...The severe acute respiratory syndrome associated coronavirus 2 (SARS-CoV-2) and SARS-CoV-1 accessory protein Orf3a colocalizes with markers of the plasma membrane, endocytic pathway, and Golgi apparatus. Some reports have led to annotation of both Orf3a proteins as viroporins. Here, we show that neither SARS-CoV-2 nor SARS-CoV-1 Orf3a form functional ion conducting pores and that the conductances measured are common contaminants in overexpression and with high levels of protein in reconstitution studies. Cryo-EM structures of both SARS-CoV-2 and SARS-CoV-1 Orf3a display a narrow constriction and the presence of a positively charged aqueous vestibule, which would not favor cation permeation. We observe enrichment of the late endosomal marker Rab7 upon SARS-CoV-2 Orf3a overexpression, and co-immunoprecipitation with VPS39. Interestingly, SARS-CoV-1 Orf3a does not cause the same cellular phenotype as SARS-CoV-2 Orf3a and does not interact with VPS39. To explain this difference, we find that a divergent, unstructured loop of SARS-CoV-2 Orf3a facilitates its binding with VPS39, a HOPS complex tethering protein involved in late endosome and autophagosome fusion with lysosomes. We suggest that the added loop enhances SARS-CoV-2 Orf3a's ability to co-opt host cellular trafficking mechanisms for viral exit or host immune evasion. #1: Journal: bioRxiv / Year: 2022 Title: The SARS-CoV-2 accessory protein Orf3a is not an ion channel, but does interact with trafficking proteins. Authors: Alexandria N Miller / Patrick R Houlihan / Ella Matamala / Deny Cabezas-Bratesco / Gi Young Lee / Ben Cristofori-Armstrong / Tanya L Dilan / Silvia Sanchez-Martinez / Doreen Matthies / Rui ...Authors: Alexandria N Miller / Patrick R Houlihan / Ella Matamala / Deny Cabezas-Bratesco / Gi Young Lee / Ben Cristofori-Armstrong / Tanya L Dilan / Silvia Sanchez-Martinez / Doreen Matthies / Rui Yan / Zhiheng Yu / Dejian Ren / Sebastian E Brauchi / David E Clapham Abstract: The severe acute respiratory syndrome associated coronavirus 2 (SARS-CoV-2) and SARS-CoV-1 accessory protein Orf3a colocalizes with markers of the plasma membrane, endocytic pathway, and Golgi ...The severe acute respiratory syndrome associated coronavirus 2 (SARS-CoV-2) and SARS-CoV-1 accessory protein Orf3a colocalizes with markers of the plasma membrane, endocytic pathway, and Golgi apparatus. Some reports have led to annotation of both Orf3a proteins as a viroporin. Here we show that neither SARS-CoV-2 nor SARS-CoV-1 form functional ion conducting pores and that the conductances measured are common contaminants in overexpression and with high levels of protein in reconstitution studies. Cryo-EM structures of both SARS-CoV-2 and SARS-CoV-1 Orf3a display a narrow constriction and the presence of a basic aqueous vestibule, which would not favor cation permeation. We observe enrichment of the late endosomal marker Rab7 upon SARS-CoV-2 Orf3a overexpression, and co-immunoprecipitation with VPS39. Interestingly, SARS-CoV-1 Orf3a does not cause the same cellular phenotype as SARS-CoV-2 Orf3a and does not interact with VPS39. To explain this difference, we find that a divergent, unstructured loop of SARS-CoV-2 Orf3a facilitates its binding with VPS39, a HOPS complex tethering protein involved in late endosome and autophagosome fusion with lysosomes. We suggest that the added loop enhances SARS-CoV-2 Orf3a ability to co-opt host cellular trafficking mechanisms for viral exit or host immune evasion. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8eqt.cif.gz 8eqt.cif.gz | 83.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8eqt.ent.gz pdb8eqt.ent.gz | 62.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8eqt.json.gz 8eqt.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/eq/8eqt https://data.pdbj.org/pub/pdb/validation_reports/eq/8eqt ftp://data.pdbj.org/pub/pdb/validation_reports/eq/8eqt ftp://data.pdbj.org/pub/pdb/validation_reports/eq/8eqt | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  28545MC  8eqjC  8eqsC  8equC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 36489.445 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Severe acute respiratory syndrome coronavirus 2 Severe acute respiratory syndrome coronavirus 2Gene: 3a / Production host:   Homo sapiens (human) / References: UniProt: P0DTC3 Homo sapiens (human) / References: UniProt: P0DTC3#2: Chemical | ChemComp-PEE /  Discrete optimized protein energy Discrete optimized protein energyHas ligand of interest | N | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Structure of SARS-CoV2 Orf3a in plasma membrane-like environment, MSP1D1 nanodisc Type: COMPLEX / Entity ID: #1 / Source: RECOMBINANT |
|---|---|
| Molecular weight | Experimental value: NO |
| Source (natural) | Organism:   Severe acute respiratory syndrome coronavirus 2 Severe acute respiratory syndrome coronavirus 2 |
| Source (recombinant) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 7.5 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2000 nm / Nominal defocus min: 800 nm Bright-field microscopy / Nominal defocus max: 2000 nm / Nominal defocus min: 800 nm |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) / Num. of grids imaged: 1 / Num. of real images: 15637 |
| EM imaging optics | Energyfilter name : GIF Bioquantum : GIF Bioquantum |
- Processing
Processing
| EM software |
| ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
CTF correction | Type: NONE | ||||||||||||||||||||
| Symmetry | Point symmetry : C2 (2 fold cyclic : C2 (2 fold cyclic ) ) | ||||||||||||||||||||
3D reconstruction | Resolution: 3.4 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 125625 / Num. of class averages: 1 / Symmetry type: POINT | ||||||||||||||||||||
| Atomic model building | Protocol: AB INITIO MODEL |
 Movie
Movie Controller
Controller



 PDBj
PDBj