[English] 日本語
 Yorodumi
Yorodumi- EMDB-28545: Structure of SARS-CoV-2 Orf3a in plasma membrane-like environment... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
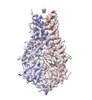
| Title | Structure of SARS-CoV-2 Orf3a in plasma membrane-like environment, MSP1D1 nanodisc | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationhost cell lysosome / induction by virus of host reticulophagy / Maturation of protein 3a / SARS-CoV-2 modulates autophagy / inorganic cation transmembrane transport / host cell endoplasmic reticulum /  voltage-gated calcium channel complex / SARS-CoV-2 targets host intracellular signalling and regulatory pathways / voltage-gated calcium channel complex / SARS-CoV-2 targets host intracellular signalling and regulatory pathways /  voltage-gated potassium channel complex / molecular function activator activity ...host cell lysosome / induction by virus of host reticulophagy / Maturation of protein 3a / SARS-CoV-2 modulates autophagy / inorganic cation transmembrane transport / host cell endoplasmic reticulum / voltage-gated potassium channel complex / molecular function activator activity ...host cell lysosome / induction by virus of host reticulophagy / Maturation of protein 3a / SARS-CoV-2 modulates autophagy / inorganic cation transmembrane transport / host cell endoplasmic reticulum /  voltage-gated calcium channel complex / SARS-CoV-2 targets host intracellular signalling and regulatory pathways / voltage-gated calcium channel complex / SARS-CoV-2 targets host intracellular signalling and regulatory pathways /  voltage-gated potassium channel complex / molecular function activator activity / : / protein complex oligomerization / monoatomic ion channel activity / host cell endosome / Translation of Structural Proteins / Virion Assembly and Release / Induction of Cell-Cell Fusion / Attachment and Entry / host cell endoplasmic reticulum membrane / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / extracellular region / identical protein binding / voltage-gated potassium channel complex / molecular function activator activity / : / protein complex oligomerization / monoatomic ion channel activity / host cell endosome / Translation of Structural Proteins / Virion Assembly and Release / Induction of Cell-Cell Fusion / Attachment and Entry / host cell endoplasmic reticulum membrane / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / extracellular region / identical protein binding /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |   Severe acute respiratory syndrome coronavirus 2 Severe acute respiratory syndrome coronavirus 2 | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.4 Å cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Miller AN / Houlihan PR / Matamala E / Cabezas-Bratesco D / Lee GY / Cristofori-Armstrong B / Dilan TL / Sanchez-Martinez S / Matthies D / Yan R ...Miller AN / Houlihan PR / Matamala E / Cabezas-Bratesco D / Lee GY / Cristofori-Armstrong B / Dilan TL / Sanchez-Martinez S / Matthies D / Yan R / Yu Z / Ren D / Brauchi SE / Clapham DE | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation | Journal: bioRxiv / Year: 2022 Title: The SARS-CoV-2 accessory protein Orf3a is not an ion channel, but does interact with trafficking proteins. Authors: Alexandria N Miller / Patrick R Houlihan / Ella Matamala / Deny Cabezas-Bratesco / Gi Young Lee / Ben Cristofori-Armstrong / Tanya L Dilan / Silvia Sanchez-Martinez / Doreen Matthies / Rui ...Authors: Alexandria N Miller / Patrick R Houlihan / Ella Matamala / Deny Cabezas-Bratesco / Gi Young Lee / Ben Cristofori-Armstrong / Tanya L Dilan / Silvia Sanchez-Martinez / Doreen Matthies / Rui Yan / Zhiheng Yu / Dejian Ren / Sebastian E Brauchi / David E Clapham Abstract: The severe acute respiratory syndrome associated coronavirus 2 (SARS-CoV-2) and SARS-CoV-1 accessory protein Orf3a colocalizes with markers of the plasma membrane, endocytic pathway, and Golgi ...The severe acute respiratory syndrome associated coronavirus 2 (SARS-CoV-2) and SARS-CoV-1 accessory protein Orf3a colocalizes with markers of the plasma membrane, endocytic pathway, and Golgi apparatus. Some reports have led to annotation of both Orf3a proteins as a viroporin. Here we show that neither SARS-CoV-2 nor SARS-CoV-1 form functional ion conducting pores and that the conductances measured are common contaminants in overexpression and with high levels of protein in reconstitution studies. Cryo-EM structures of both SARS-CoV-2 and SARS-CoV-1 Orf3a display a narrow constriction and the presence of a basic aqueous vestibule, which would not favor cation permeation. We observe enrichment of the late endosomal marker Rab7 upon SARS-CoV-2 Orf3a overexpression, and co-immunoprecipitation with VPS39. Interestingly, SARS-CoV-1 Orf3a does not cause the same cellular phenotype as SARS-CoV-2 Orf3a and does not interact with VPS39. To explain this difference, we find that a divergent, unstructured loop of SARS-CoV-2 Orf3a facilitates its binding with VPS39, a HOPS complex tethering protein involved in late endosome and autophagosome fusion with lysosomes. We suggest that the added loop enhances SARS-CoV-2 Orf3a ability to co-opt host cellular trafficking mechanisms for viral exit or host immune evasion. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28545.map.gz emd_28545.map.gz | 4.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28545-v30.xml emd-28545-v30.xml emd-28545.xml emd-28545.xml | 17.9 KB 17.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_28545.png emd_28545.png | 67 KB | ||
| Others |  emd_28545_half_map_1.map.gz emd_28545_half_map_1.map.gz emd_28545_half_map_2.map.gz emd_28545_half_map_2.map.gz | 48.6 MB 48.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28545 http://ftp.pdbj.org/pub/emdb/structures/EMD-28545 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28545 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28545 | HTTPS FTP |
-Related structure data
| Related structure data |  8eqtMC  8eqjC  8eqsC  8equC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_28545.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28545.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.844 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_28545_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_28545_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Structure of SARS-CoV2 Orf3a in plasma membrane-like environment,...
| Entire | Name: Structure of SARS-CoV2 Orf3a in plasma membrane-like environment, MSP1D1 nanodisc |
|---|---|
| Components |
|
-Supramolecule #1: Structure of SARS-CoV2 Orf3a in plasma membrane-like environment,...
| Supramolecule | Name: Structure of SARS-CoV2 Orf3a in plasma membrane-like environment, MSP1D1 nanodisc type: complex / ID: 1 / Chimera: Yes / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Severe acute respiratory syndrome coronavirus 2 Severe acute respiratory syndrome coronavirus 2 |
-Macromolecule #1: ORF3a protein
| Macromolecule | Name: ORF3a protein / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Severe acute respiratory syndrome coronavirus 2 Severe acute respiratory syndrome coronavirus 2 |
| Molecular weight | Theoretical: 36.489445 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MDLFMRIFTI GTVTLKQGEI KDATPSDFVR ATATIPIQAS LPFGWLIVGV ALLAVFQSAS KIITLKKRWQ LALSKGVHFV CNLLLLFVT VYSHLLLVAA GLEAPFLYLY ALVYFLQSIN FVRIIMRLWL CWKCRSKNPL LYDANYFLCW HTNCYDYCIP Y NSVTSSIV ...String: MDLFMRIFTI GTVTLKQGEI KDATPSDFVR ATATIPIQAS LPFGWLIVGV ALLAVFQSAS KIITLKKRWQ LALSKGVHFV CNLLLLFVT VYSHLLLVAA GLEAPFLYLY ALVYFLQSIN FVRIIMRLWL CWKCRSKNPL LYDANYFLCW HTNCYDYCIP Y NSVTSSIV ITSGDGTTSP ISEHDYQIGG YTEKWESGVK DCVVLHSYFT SDYYQLYSTQ LSTDTGVEHV TFFIYNKIVD EP EEHVQIH TIDGSSGVVN PVMEPIYDEP TTTTSVPLGG RGLEVLFQGP GSGQLVGSGG LEGGGGWSHP QFEKGGGSGG GSG GGSWSH PQFEK |
-Macromolecule #2: 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
| Macromolecule | Name: 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine / type: ligand / ID: 2 / Number of copies: 4 / Formula: PEE |
|---|---|
| Molecular weight | Theoretical: 744.034 Da |
| Chemical component information | 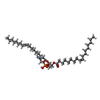 ChemComp-PEE: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm Bright-field microscopy / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm |
| Specialist optics | Energy filter - Name: GIF Bioquantum |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 15637 / Average electron dose: 50.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: cryoSPARC (ver. 3.0) |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: RELION (ver. 3.1) |
| Final reconstruction | Number classes used: 1 / Applied symmetry - Point group: C2 (2 fold cyclic ) / Resolution.type: BY AUTHOR / Resolution: 3.4 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 125625 ) / Resolution.type: BY AUTHOR / Resolution: 3.4 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 125625 |
-Atomic model buiding 1
| Refinement | Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-8eqt: |
 Movie
Movie Controller
Controller




 Z
Z Y
Y X
X


















