[English] 日本語
 Yorodumi
Yorodumi- PDB-8att: Cryo-EM structure of yeast mitochondrial RNA polymerase transcrip... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8att | ||||||
|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of yeast mitochondrial RNA polymerase transcription initiation complex with 4-mer RNA, pppGpGpUpA (IC4) | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  TRANSCRIPTION / TRANSCRIPTION /  gene transcription / gene transcription /  polymerase / polymerase /  RDRP / MTF1 / RPO41 / RDRP / MTF1 / RPO41 /  POLRMT / mtRNAP / POLRMT / mtRNAP /  DNA / DNA /  transcription initiation / transcription initiation /  RNA polymerase / RNA polymerase /  mitochondria mitochondria | ||||||
| Function / homology |  Function and homology information Function and homology informationMitochondrial transcription initiation / mitochondrial DNA-directed RNA polymerase complex / mitochondrial promoter sequence-specific DNA binding / mitochondrial transcription factor activity /  transcription initiation at mitochondrial promoter / mitochondrial transcription / transcription initiation at mitochondrial promoter / mitochondrial transcription /  mitochondrial genome maintenance / mitochondrial genome maintenance /  DNA primase activity / DNA primase activity /  DNA replication, synthesis of primer / positive regulation of DNA-templated transcription, elongation ...Mitochondrial transcription initiation / mitochondrial DNA-directed RNA polymerase complex / mitochondrial promoter sequence-specific DNA binding / mitochondrial transcription factor activity / DNA replication, synthesis of primer / positive regulation of DNA-templated transcription, elongation ...Mitochondrial transcription initiation / mitochondrial DNA-directed RNA polymerase complex / mitochondrial promoter sequence-specific DNA binding / mitochondrial transcription factor activity /  transcription initiation at mitochondrial promoter / mitochondrial transcription / transcription initiation at mitochondrial promoter / mitochondrial transcription /  mitochondrial genome maintenance / mitochondrial genome maintenance /  DNA primase activity / DNA primase activity /  DNA replication, synthesis of primer / positive regulation of DNA-templated transcription, elongation / mitochondrial nucleoid / DNA replication, synthesis of primer / positive regulation of DNA-templated transcription, elongation / mitochondrial nucleoid /  Transferases; Transferring one-carbon groups; Methyltransferases / Transferases; Transferring one-carbon groups; Methyltransferases /  methyltransferase activity / methyltransferase activity /  mitochondrial intermembrane space / DNA-directed 5'-3' RNA polymerase activity / mitochondrial intermembrane space / DNA-directed 5'-3' RNA polymerase activity /  DNA-directed RNA polymerase / DNA-directed RNA polymerase /  methylation / methylation /  mitochondrial matrix / mitochondrial matrix /  mitochondrion / mitochondrion /  DNA binding / DNA binding /  RNA binding / RNA binding /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Saccharomyces cerevisiae S288C (yeast) Saccharomyces cerevisiae S288C (yeast) | ||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.44 Å cryo EM / Resolution: 3.44 Å | ||||||
 Authors Authors | Goovaerts, Q. / Shen, J. / Patel, S.S. / Das, K. | ||||||
| Funding support |  Belgium, 1items Belgium, 1items
| ||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Structures illustrate step-by-step mitochondrial transcription initiation. Authors: Quinten Goovaerts / Jiayu Shen / Brent De Wijngaert / Urmimala Basu / Smita S Patel / Kalyan Das /   Abstract: Transcription initiation is a key regulatory step in gene expression during which RNA polymerase (RNAP) initiates RNA synthesis de novo, and the synthesized RNA at a specific length triggers the ...Transcription initiation is a key regulatory step in gene expression during which RNA polymerase (RNAP) initiates RNA synthesis de novo, and the synthesized RNA at a specific length triggers the transition to the elongation phase. Mitochondria recruit a single-subunit RNAP and one or two auxiliary factors to initiate transcription. Previous studies have revealed the molecular architectures of yeast and human mitochondrial RNAP initiation complexes (ICs). Here we provide a comprehensive, stepwise mechanism of transcription initiation by solving high-resolution cryogenic electron microscopy (cryo-EM) structures of yeast mitochondrial RNAP and the transcription factor Mtf1 catalysing two- to eight-nucleotide RNA synthesis at single-nucleotide addition steps. The growing RNA-DNA is accommodated in the polymerase cleft by template scrunching and non-template reorganization, creating stressed intermediates. During early initiation, non-template strand scrunching and unscrunching destabilize the short two- and three-nucleotide RNAs, triggering abortive synthesis. Subsequently, the non-template reorganizes into a base-stacked staircase-like structure supporting processive five- to eight-nucleotide RNA synthesis. The expanded non-template staircase and highly scrunched template in IC8 destabilize the promoter interactions with Mtf1 to facilitate initiation bubble collapse and promoter escape for the transition from initiation to the elongation complex (EC). The series of transcription initiation steps, each guided by the interplay of multiple structural components, reveal a finely tuned mechanism for potential regulatory control. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8att.cif.gz 8att.cif.gz | 274.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8att.ent.gz pdb8att.ent.gz | 205.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8att.json.gz 8att.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/at/8att https://data.pdbj.org/pub/pdb/validation_reports/at/8att ftp://data.pdbj.org/pub/pdb/validation_reports/at/8att ftp://data.pdbj.org/pub/pdb/validation_reports/at/8att | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  15662MC  8ap1C  8atvC  8atwC  8c5sC  8c5uC  8q63C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 2 types, 2 molecules BA
| #1: Protein | Mass: 41151.203 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Saccharomyces cerevisiae S288C (yeast) / Strain: ATCC 204508 / S288c / Gene: MTF1, YMR228W, YM9959.10 / Plasmid: pTrcHisC / Production host: Saccharomyces cerevisiae S288C (yeast) / Strain: ATCC 204508 / S288c / Gene: MTF1, YMR228W, YM9959.10 / Plasmid: pTrcHisC / Production host:   Escherichia coli BL21(DE3) (bacteria) Escherichia coli BL21(DE3) (bacteria)References: UniProt: P14908,  Transferases; Transferring one-carbon groups; Methyltransferases Transferases; Transferring one-carbon groups; Methyltransferases |
|---|---|
| #2: Protein |  Polymerase PolymeraseMass: 143282.656 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: Residues 101-1351 / Source: (gene. exp.)   Saccharomyces cerevisiae S288C (yeast) / Strain: ATCC 204508 / S288c / Gene: RPO41, YFL036W / Plasmid: ProEXHTB / Production host: Saccharomyces cerevisiae S288C (yeast) / Strain: ATCC 204508 / S288c / Gene: RPO41, YFL036W / Plasmid: ProEXHTB / Production host:   Escherichia coli BL21(DE3) (bacteria) / Variant (production host): CodonPlus RIL / References: UniProt: P13433, Escherichia coli BL21(DE3) (bacteria) / Variant (production host): CodonPlus RIL / References: UniProt: P13433,  DNA-directed RNA polymerase DNA-directed RNA polymerase |
-DNA chain , 2 types, 2 molecules NT
| #3: DNA chain | Mass: 10248.671 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.)   Saccharomyces cerevisiae S288C (yeast) Saccharomyces cerevisiae S288C (yeast) |
|---|---|
| #4: DNA chain | Mass: 10047.511 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.)   Saccharomyces cerevisiae S288C (yeast) Saccharomyces cerevisiae S288C (yeast) |
-RNA chain / Non-polymers , 2 types, 2 molecules C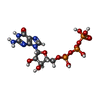

| #5: RNA chain | Mass: 935.620 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.)   Saccharomyces cerevisiae S288C (yeast) Saccharomyces cerevisiae S288C (yeast) |
|---|---|
| #6: Chemical | ChemComp-GTP /  Guanosine triphosphate Guanosine triphosphate |
-Details
| Has ligand of interest | N |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight |
| ||||||||||||||||||||||||||||||||||||||||||
| Source (natural) |
| ||||||||||||||||||||||||||||||||||||||||||
| Source (recombinant) |
| ||||||||||||||||||||||||||||||||||||||||||
| Buffer solution | pH: 7 Details: 50 mM Bis-tris propane, 100 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 2 mM DTT | ||||||||||||||||||||||||||||||||||||||||||
| Specimen | Conc.: 0.7 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES | ||||||||||||||||||||||||||||||||||||||||||
| Specimen support | Grid material: GOLD / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R1.2/1.3 | ||||||||||||||||||||||||||||||||||||||||||
Vitrification | Instrument: LEICA EM GP / Cryogen name: ETHANE / Humidity: 95 % / Chamber temperature: 281.15 K / Details: 3 uL sample, back blotting for 12 seconds |
- Electron microscopy imaging
Electron microscopy imaging
| Microscopy | Model: TFS GLACIOS |
|---|---|
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal magnification: 150000 X / Nominal defocus max: 1800 nm / Nominal defocus min: 800 nm / Calibrated defocus min: 550 nm / Calibrated defocus max: 2600 nm / Cs Bright-field microscopy / Nominal magnification: 150000 X / Nominal defocus max: 1800 nm / Nominal defocus min: 800 nm / Calibrated defocus min: 550 nm / Calibrated defocus max: 2600 nm / Cs : 2.7 mm / C2 aperture diameter: 50 µm / Alignment procedure: COMA FREE : 2.7 mm / C2 aperture diameter: 50 µm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: OTHER |
| Image recording | Average exposure time: 38.41 sec. / Electron dose: 40 e/Å2 / Detector mode: COUNTING / Film or detector model: FEI FALCON III (4k x 4k) / Num. of grids imaged: 1 / Num. of real images: 997 |
| EM imaging optics | Energyfilter slit width: 20 eV |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
CTF correction | Type: NONE | ||||||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 671969 | ||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry : C1 (asymmetric) : C1 (asymmetric) | ||||||||||||||||||||||||||||||||||||||||
3D reconstruction | Resolution: 3.44 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 138730 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||
| Atomic model building | B value: 80 / Protocol: FLEXIBLE FIT / Space: REAL / Target criteria: Correlation coefficient / Details: Realspace refinement | ||||||||||||||||||||||||||||||||||||||||
| Atomic model building | PDB-ID: 6YMV Accession code: 6YMV / Source name: PDB / Type: experimental model |
 Movie
Movie Controller
Controller










 PDBj
PDBj







































