[English] 日本語
 Yorodumi
Yorodumi- PDB-6zyx: Outer Dynein Arm-Shulin complex - Shulin region from Tetrahymena ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6zyx | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Outer Dynein Arm-Shulin complex - Shulin region from Tetrahymena thermophila | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords |  MOTOR PROTEIN / MOTOR PROTEIN /  Cilia / Cilia /  Microtubules / Microtubules /  Motor / Motor /  Complex Complex | |||||||||
| Function / homology |  Function and homology information Function and homology information dynein complex / minus-end-directed microtubule motor activity / dynein light intermediate chain binding / dynein complex / minus-end-directed microtubule motor activity / dynein light intermediate chain binding /  motile cilium / dynein intermediate chain binding / microtubule-based movement / microtubule-based process / motile cilium / dynein intermediate chain binding / microtubule-based movement / microtubule-based process /  microtubule / microtubule /  ATP hydrolysis activity / ATP hydrolysis activity /  ATP binding / ATP binding /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Tetrahymena thermophila CU428 (eukaryote) Tetrahymena thermophila CU428 (eukaryote) | |||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 4.3 Å cryo EM / Resolution: 4.3 Å | |||||||||
 Authors Authors | Mali, G.R. / Abid Ali, F. / Lau, C.K. / Carter, A.P. | |||||||||
| Funding support |  United Kingdom, 2items United Kingdom, 2items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2021 Journal: Science / Year: 2021Title: Shulin packages axonemal outer dynein arms for ciliary targeting. Authors: Girish R Mali / Ferdos Abid Ali / Clinton K Lau / Farida Begum / Jérôme Boulanger / Jonathan D Howe / Zhuo A Chen / Juri Rappsilber / Mark Skehel / Andrew P Carter /   Abstract: The main force generators in eukaryotic cilia and flagella are axonemal outer dynein arms (ODAs). During ciliogenesis, these ~1.8-megadalton complexes are assembled in the cytoplasm and targeted to ...The main force generators in eukaryotic cilia and flagella are axonemal outer dynein arms (ODAs). During ciliogenesis, these ~1.8-megadalton complexes are assembled in the cytoplasm and targeted to cilia by an unknown mechanism. Here, we used the ciliate to identify two factors (Q22YU3 and Q22MS1) that bind ODAs in the cytoplasm and are required for ODA delivery to cilia. Q22YU3, which we named Shulin, locked the ODA motor domains into a closed conformation and inhibited motor activity. Cryo-electron microscopy revealed how Shulin stabilized this compact form of ODAs by binding to the dynein tails. Our findings provide a molecular explanation for how newly assembled dyneins are packaged for delivery to the cilia. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6zyx.cif.gz 6zyx.cif.gz | 496.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6zyx.ent.gz pdb6zyx.ent.gz | 330.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6zyx.json.gz 6zyx.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/zy/6zyx https://data.pdbj.org/pub/pdb/validation_reports/zy/6zyx ftp://data.pdbj.org/pub/pdb/validation_reports/zy/6zyx ftp://data.pdbj.org/pub/pdb/validation_reports/zy/6zyx | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  11579MC  6zywC  6zyyC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 4 types, 4 molecules CYde
| #1: Protein | Mass: 534328.812 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila CU428 (eukaryote) / References: UniProt: Q22A67 Tetrahymena thermophila CU428 (eukaryote) / References: UniProt: Q22A67 |
|---|---|
| #8: Protein |  Shulin District Shulin DistrictMass: 139935.781 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Tetrahymena thermophila CU428 (eukaryote) Tetrahymena thermophila CU428 (eukaryote)Gene: TTHERM_00122270 / Production host:   Spodoptera frugiperda (fall armyworm) / References: UniProt: Q22YU3 Spodoptera frugiperda (fall armyworm) / References: UniProt: Q22YU3 |
| #9: Protein | Mass: 77888.219 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila CU428 (eukaryote) / References: UniProt: I7M008 Tetrahymena thermophila CU428 (eukaryote) / References: UniProt: I7M008 |
| #10: Protein | Mass: 77178.062 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila CU428 (eukaryote) / References: UniProt: Q23FU1 Tetrahymena thermophila CU428 (eukaryote) / References: UniProt: Q23FU1 |
-Dynein light ... , 6 types, 6 molecules JKLMNO
| #2: Protein |  Mass: 10973.408 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila CU428 (eukaryote) / References: UniProt: Q24DI9 Tetrahymena thermophila CU428 (eukaryote) / References: UniProt: Q24DI9 |
|---|---|
| #3: Protein |  Mass: 13336.089 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila CU428 (eukaryote) / References: UniProt: Q22R86 Tetrahymena thermophila CU428 (eukaryote) / References: UniProt: Q22R86 |
| #4: Protein |  Mass: 12516.457 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila CU428 (eukaryote) / References: UniProt: W7XJB1 Tetrahymena thermophila CU428 (eukaryote) / References: UniProt: W7XJB1 |
| #5: Protein |  Mass: 10453.167 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila CU428 (eukaryote) / References: UniProt: Q24CE5 Tetrahymena thermophila CU428 (eukaryote) / References: UniProt: Q24CE5 |
| #6: Protein | Mass: 15608.120 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila CU428 (eukaryote) / References: UniProt: Q1HGH8 Tetrahymena thermophila CU428 (eukaryote) / References: UniProt: Q1HGH8 |
| #7: Protein | Mass: 13202.817 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila CU428 (eukaryote) / References: UniProt: A4VEB3 Tetrahymena thermophila CU428 (eukaryote) / References: UniProt: A4VEB3 |
-Non-polymers , 1 types, 1 molecules 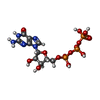
| #11: Chemical | ChemComp-GTP /  Guanosine triphosphate Guanosine triphosphate |
|---|
-Details
| Has ligand of interest | N |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Experimental value: NO | ||||||||||||||||||||||||
| Source (natural) |
| ||||||||||||||||||||||||
| Source (recombinant) | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) | ||||||||||||||||||||||||
| Buffer solution | pH: 7.4 | ||||||||||||||||||||||||
| Specimen | Conc.: 0.2 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES | ||||||||||||||||||||||||
Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Microscopy | Model: FEI TITAN |
|---|---|
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy Bright-field microscopy |
| Image recording | Electron dose: 52 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: dev_3965: / Classification: refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
3D reconstruction | Resolution: 4.3 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 43338 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller

















 PDBj
PDBj


