[English] 日本語
 Yorodumi
Yorodumi- PDB-1cwz: Solution structure of the analogue retro-inverso (MA-S)REGRIGGC i... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1cwz | ||||||
|---|---|---|---|---|---|---|---|
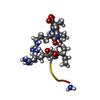
| Title | Solution structure of the analogue retro-inverso (MA-S)REGRIGGC in contact with the monoclonal antibody MAB 4X11, NMR, 7 structures | ||||||
 Components Components | HISTONE H3 | ||||||
 Keywords Keywords |  DNA BINDING PROTEIN / PSEUDOMIMETIC / DNA BINDING PROTEIN / PSEUDOMIMETIC /  SYNTHETIC PEPTIDE / RETRO-INVERSO ANALOGUE / TR-NOE / ANTIGEN- ANTIBODY COMPLEX SYNTHETIC PEPTIDE / RETRO-INVERSO ANALOGUE / TR-NOE / ANTIGEN- ANTIBODY COMPLEX | ||||||
| Function / homology |  METHYLMALONIC ACID METHYLMALONIC ACID Function and homology information Function and homology information | ||||||
| Method |  SOLUTION NMR / ENERGY MINIMISATION MOLECULAR DYNAMICS (SIMULATED ANNEALING) SOLUTION NMR / ENERGY MINIMISATION MOLECULAR DYNAMICS (SIMULATED ANNEALING) | ||||||
 Authors Authors | Phan Chan Du, A. / Petit, M.C. / Guichard, G. / Briand, J.P. / Muller, S. / Cung, M.T. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2001 Journal: Biochemistry / Year: 2001Title: Structure of antibody-bound peptides and retro-inverso analogues. A transferred nuclear Overhauser effect spectroscopy and molecular dynamics approach. Authors: Phan-Chan-Du, A. / Petit, M.C. / Guichard, G. / Briand, J.P. / Muller, S. / Cung, M.T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1cwz.cif.gz 1cwz.cif.gz | 22.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1cwz.ent.gz pdb1cwz.ent.gz | 16.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1cwz.json.gz 1cwz.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/cw/1cwz https://data.pdbj.org/pub/pdb/validation_reports/cw/1cwz ftp://data.pdbj.org/pub/pdb/validation_reports/cw/1cwz ftp://data.pdbj.org/pub/pdb/validation_reports/cw/1cwz | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein/peptide |  Mass: 846.980 Da / Num. of mol.: 1 / Fragment: C-TERMINAL DOMAIN: Residues 130-135 / Source method: obtained synthetically Details: THE PEPTIDE WAS CHEMICALLY SYNTHESIZED. THE SEQUENCE CGG WAS ADDED AS A LINKER TO THE DEXTRAN MATRIX IN BIACORE EXPERIMENTS VIA THE CYS THIOL GROUP. NH2-CO WAS ADDED AT THE C-TERMINAL ...Details: THE PEPTIDE WAS CHEMICALLY SYNTHESIZED. THE SEQUENCE CGG WAS ADDED AS A LINKER TO THE DEXTRAN MATRIX IN BIACORE EXPERIMENTS VIA THE CYS THIOL GROUP. NH2-CO WAS ADDED AT THE C-TERMINAL EXTREMITY OF THE RETRO-INVERSO PEPTIDE IN ORDER TO MIMIC THE N-TERMINAL OF THE PARENT PEPTIDE. ALL NON GLYCINE RESIDUE PRESENT A D-CONFIGURATION EXCEPT CYS. TWO RI(MA) DIASTEREISOMERS WERE OBTAINED AND COULDN'T BE SEPARATED. |
|---|---|
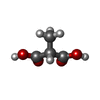
| #2: Chemical | ChemComp-DXX /  Methylmalonic acid Methylmalonic acid |
-Experimental details
-Experiment
| Experiment | Method:  SOLUTION NMR SOLUTION NMR |
|---|---|
| NMR experiment | Type : :  COSY, TOCSY, COSY, TOCSY,  NOESY NOESY |
| NMR details | Text: THE PEPTIDE/MAB MOLAR RATIO WAS ADJUSTED TO 50/1(I.E. 5 MM OF PEPTIDE AND 0.1 MM OF MAB) |
- Sample preparation
Sample preparation
| Details | Contents: 5MM PEPTIDE, 0.1 MM MAB. 100 MM PHOSPHATE BUFFER CONTAINING 0.02% SODIUM AZIDE |
|---|---|
| Sample conditions | Ionic strength: 0.1M PHOSPHATE / pH: 7 / Pressure: 1 atm / Temperature: 277 K |
Crystal grow | *PLUS Method: other / Details: NMR |
-NMR measurement
| NMR spectrometer | Type: Bruker DRX / Manufacturer: Bruker / Model : DRX / Field strength: 400 MHz : DRX / Field strength: 400 MHz |
|---|
- Processing
Processing
| NMR software |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: ENERGY MINIMISATION MOLECULAR DYNAMICS (SIMULATED ANNEALING) Software ordinal: 1 Details: THE STRUCTURES ARE BASED ON A SET OF 35 TO 60 BACKBONE-BACKBONE, BACKBONE-SIDE CHAIN AND SIDE CHAIN-SIDE CHAIN DISTANCE RESTRAINTS. THE PHI ANGLE FOR THE NON GLYCINE D-RESIDUES WAS ...Details: THE STRUCTURES ARE BASED ON A SET OF 35 TO 60 BACKBONE-BACKBONE, BACKBONE-SIDE CHAIN AND SIDE CHAIN-SIDE CHAIN DISTANCE RESTRAINTS. THE PHI ANGLE FOR THE NON GLYCINE D-RESIDUES WAS CONSTRAINED BETWEEN 0 AND 175. A DISTANCE DEPENDENT DIELECTRIC CONSTANT EQUAL TO 4R WAS APPLIED. THE NET ELECTRIC CHARGES WERE DECREASED, WHILE THOSE OF THE N AND C-TERMINAL CHARGED GROUPS WERE NEGLECTED. | ||||||||||||||||
| NMR representative | Selection criteria: lowest energy | ||||||||||||||||
| NMR ensemble | Conformer selection criteria: structures with the lowest energy Conformers calculated total number: 50 / Conformers submitted total number: 7 |
 Movie
Movie Controller
Controller










 PDBj
PDBj