[English] 日本語
 Yorodumi
Yorodumi- EMDB-41088: SpRY-Cas9:gRNA complex bound to non-target DNA with 10 bp R-loop -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | SpRY-Cas9:gRNA complex bound to non-target DNA with 10 bp R-loop | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | SpRY-Cas9 /  CRISPR / CRISPR /  Cas9 / Cas9 /  IMMUNE SYSTEM / IMMUNE SYSTEM /  R-loop R-loop | |||||||||
| Function / homology |  Function and homology information Function and homology informationmaintenance of CRISPR repeat elements / 3'-5' exonuclease activity / DNA endonuclease activity / defense response to virus /  Hydrolases; Acting on ester bonds / Hydrolases; Acting on ester bonds /  DNA binding / DNA binding /  RNA binding / RNA binding /  metal ion binding metal ion bindingSimilarity search - Function | |||||||||
| Biological species |   Streptococcus pyogenes (bacteria) / Streptococcus pyogenes (bacteria) /   Escherichia phage Lambda (virus) Escherichia phage Lambda (virus) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.04 Å cryo EM / Resolution: 3.04 Å | |||||||||
 Authors Authors | Hibshman GN / Bravo JPK / Taylor DW | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Unraveling the mechanisms of PAMless DNA interrogation by SpRY-Cas9. Authors: Grace N Hibshman / Jack P K Bravo / Matthew M Hooper / Tyler L Dangerfield / Hongshan Zhang / Ilya J Finkelstein / Kenneth A Johnson / David W Taylor /   Abstract: CRISPR-Cas9 is a powerful tool for genome editing, but the strict requirement for an NGG protospacer-adjacent motif (PAM) sequence immediately next to the DNA target limits the number of editable ...CRISPR-Cas9 is a powerful tool for genome editing, but the strict requirement for an NGG protospacer-adjacent motif (PAM) sequence immediately next to the DNA target limits the number of editable genes. Recently developed Cas9 variants have been engineered with relaxed PAM requirements, including SpG-Cas9 (SpG) and the nearly PAM-less SpRY-Cas9 (SpRY). However, the molecular mechanisms of how SpRY recognizes all potential PAM sequences remains unclear. Here, we combine structural and biochemical approaches to determine how SpRY interrogates DNA and recognizes target sites. Divergent PAM sequences can be accommodated through conformational flexibility within the PAM-interacting region, which facilitates tight binding to off-target DNA sequences. Nuclease activation occurs ~1000-fold slower than for Streptococcus pyogenes Cas9, enabling us to directly visualize multiple on-pathway intermediate states. Experiments with SpG position it as an intermediate enzyme between Cas9 and SpRY. Our findings shed light on the molecular mechanisms of PAMless genome editing. | |||||||||
| History |
|
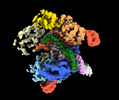
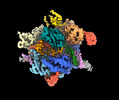
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41088.map.gz emd_41088.map.gz | 108.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41088-v30.xml emd-41088-v30.xml emd-41088.xml emd-41088.xml | 17.1 KB 17.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_41088.png emd_41088.png | 90.5 KB | ||
| Filedesc metadata |  emd-41088.cif.gz emd-41088.cif.gz | 6.8 KB | ||
| Others |  emd_41088_half_map_1.map.gz emd_41088_half_map_1.map.gz emd_41088_half_map_2.map.gz emd_41088_half_map_2.map.gz | 200.7 MB 200.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41088 http://ftp.pdbj.org/pub/emdb/structures/EMD-41088 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41088 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41088 | HTTPS FTP |
-Related structure data
| Related structure data |  8t79MC  8spqC  8sqhC  8srsC  8t6oC  8t6pC  8t6sC  8t6tC  8t6xC  8t6yC  8t76C  8t77C  8t78C  8t7sC  8tzzC  8u3yC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_41088.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41088.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8332 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_41088_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_41088_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Ternary complex of SpRY-Cas9 with gRNA and non-target DNA with 10...
| Entire | Name: Ternary complex of SpRY-Cas9 with gRNA and non-target DNA with 10 bp R-loop |
|---|---|
| Components |
|
-Supramolecule #1: Ternary complex of SpRY-Cas9 with gRNA and non-target DNA with 10...
| Supramolecule | Name: Ternary complex of SpRY-Cas9 with gRNA and non-target DNA with 10 bp R-loop type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Streptococcus pyogenes (bacteria) Streptococcus pyogenes (bacteria) |
| Molecular weight | Theoretical: 211.54 KDa |
-Macromolecule #1: CRISPR-associated endonuclease Cas9/Csn1
| Macromolecule | Name: CRISPR-associated endonuclease Cas9/Csn1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number:  Hydrolases; Acting on ester bonds Hydrolases; Acting on ester bonds |
|---|---|
| Source (natural) | Organism:   Streptococcus pyogenes (bacteria) Streptococcus pyogenes (bacteria) |
| Molecular weight | Theoretical: 158.676031 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: KKYSIGLDIG TNSVGWAVIT DEYKVPSKKF KVLGNTDRHS IKKNLIGALL FDSGETAERT RLKRTARRRY TRRKNRICYL QEIFSNEMA KVDDSFFHRL EESFLVEEDK KHERHPIFGN IVDEVAYHEK YPTIYHLRKK LVDSTDKADL RLIYLALAHM I KFRGHFLI ...String: KKYSIGLDIG TNSVGWAVIT DEYKVPSKKF KVLGNTDRHS IKKNLIGALL FDSGETAERT RLKRTARRRY TRRKNRICYL QEIFSNEMA KVDDSFFHRL EESFLVEEDK KHERHPIFGN IVDEVAYHEK YPTIYHLRKK LVDSTDKADL RLIYLALAHM I KFRGHFLI EGDLNPDNSD VDKLFIQLVQ TYNQLFEENP INASGVDAKA ILSARLSKSR RLENLIAQLP GEKKNGLFGN LI ALSLGLT PNFKSNFDLA EDAKLQLSKD TYDDDLDNLL AQIGDQYADL FLAAKNLSDA ILLSDILRVN TEITKAPLSA SMI KRYDEH HQDLTLLKAL VRQQLPEKYK EIFFDQSKNG YAGYIDGGAS QEEFYKFIKP ILEKMDGTEE LLVKLNREDL LRKQ RTFDN GSIPHQIHLG ELHAILRRQE DFYPFLKDNR EKIEKILTFR IPYYVGPLAR GNSRFAWMTR KSEETITPWN FEEVV DKGA SAQSFIERMT NFDKNLPNEK VLPKHSLLYE YFTVYNELTK VKYVTEGMRK PAFLSGEQKK AIVDLLFKTN RKVTVK QLK EDYFKKIECF DSVEISGVED RFNASLGTYH DLLKIIKDKD FLDNEENEDI LEDIVLTLTL FEDREMIEER LKTYAHL FD DKVMKQLKRR RYTGWGRLSR KLINGIRDKQ SGKTILDFLK SDGFANRNFM QLIHDDSLTF KEDIQKAQVS GQGDSLHE H IANLAGSPAI KKGILQTVKV VDELVKVMGR HKPENIVIEM ARENQTTQKG QKNSRERMKR IEEGIKELGS QILKEHPVE NTQLQNEKLY LYYLQNGRDM YVDQELDINR LSDYDVDHIV PQSFLKDDSI DNKVLTRSDK NRGKSDNVPS EEVVKKMKNY WRQLLNAKL ITQRKFDNLT KAERGGLSEL DKAGFIKRQL VETRQITKHV AQILDSRMNT KYDENDKLIR EVKVITLKSK L VSDFRKDF QFYKVREINN YHHAHDAYLN AVVGTALIKK YPKLESEFVY GDYKVYDVRK MIAKSEQEIG KATAKYFFYS NI MNFFKTE ITLANGEIRK RPLIETNGET GEIVWDKGRD FATVRKVLSM PQVNIVKKTE VQTGGFSKES IRPKRNSDKL IAR KKDWDP KKYGGFLWPT VAYSVLVVAK VEKGKSKKLK SVKELLGITI MERSSFEKNP IDFLEAKGYK EVKKDLIIKL PKYS LFELE NGRKRMLASA KQLQKGNELA LPSKYVNFLY LASHYEKLKG SPEDNEQKQL FVEQHKHYLD EIIEQISEFS KRVIL ADAN LDKVLSAYNK HRDKPIREQA ENIIHLFTLT RLGAPRAFKY FDTTIDPKQY RSTKEVLDAT LIHQSITGLY ETRIDL SQL G UniProtKB: CRISPR-associated endonuclease Cas9/Csn1 |
-Macromolecule #2: gRNA
| Macromolecule | Name: gRNA / type: rna / ID: 2 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:   Streptococcus pyogenes (bacteria) Streptococcus pyogenes (bacteria) |
| Molecular weight | Theoretical: 28.724102 KDa |
| Sequence | String: UAGAAAUACG CGUUUUAGAG CUAGAAAUAG CAAGUUAAAA UAAGGCUAGU CCGUUAUCAA CUUGAAAAAG UGGCACCGAG UCGGUGCUU |
-Macromolecule #3: TS
| Macromolecule | Name: TS / type: dna / ID: 3 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:   Escherichia phage Lambda (virus) Escherichia phage Lambda (virus) |
| Molecular weight | Theoretical: 6.032886 KDa |
| Sequence | String: (DT)(DC)(DT)(DC)(DT)(DG)(DC)(DT)(DC)(DT) (DG)(DC)(DG)(DT)(DA)(DT)(DT)(DT)(DC)(DT) |
-Macromolecule #4: NTS
| Macromolecule | Name: NTS / type: dna / ID: 4 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:   Escherichia phage Lambda (virus) Escherichia phage Lambda (virus) |
| Molecular weight | Theoretical: 2.813875 KDa |
| Sequence | String: (DG)(DA)(DG)(DC)(DA)(DG)(DA)(DG)(DA) |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.5 µm Bright-field microscopy / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.5 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 80.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.04 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 123454 |
 Movie
Movie Controller
Controller





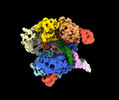





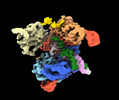

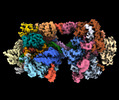



 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)