[English] 日本語
 Yorodumi
Yorodumi- EMDB-40606: Cryo-EM structure of the human nucleosome core particle in comple... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
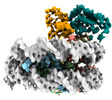
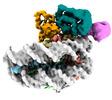
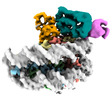
| Title | Cryo-EM structure of the human nucleosome core particle in complex with RNF168 and UbcH5c~Ub (UbcH5c chemically conjugated to histone H2A. No density for Ub.) (class 3) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  Nucleosome core particle / Nucleosome core particle /  chromatin / RNF168 / chromatin / RNF168 /  RING domain / UbcH5c / RING domain / UbcH5c /  DNA repair / DNA double-strand break / DNA repair / DNA double-strand break /  Homologous recombination / Homologous recombination /  53BP1 / 53BP1 /  ubiquitin / ubiquitin /  STRUCTURAL PROTEIN-DNA-TRANSFERASE complex STRUCTURAL PROTEIN-DNA-TRANSFERASE complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationhistone H2AK15 ubiquitin ligase activity / histone ubiquitin ligase activity / (E3-independent) E2 ubiquitin-conjugating enzyme / protein K6-linked ubiquitination / Signaling by BMP / double-strand break repair via classical nonhomologous end joining /  isotype switching / protein K11-linked ubiquitination / DNA repair-dependent chromatin remodeling / positive regulation of protein targeting to mitochondrion ...histone H2AK15 ubiquitin ligase activity / histone ubiquitin ligase activity / (E3-independent) E2 ubiquitin-conjugating enzyme / protein K6-linked ubiquitination / Signaling by BMP / double-strand break repair via classical nonhomologous end joining / isotype switching / protein K11-linked ubiquitination / DNA repair-dependent chromatin remodeling / positive regulation of protein targeting to mitochondrion ...histone H2AK15 ubiquitin ligase activity / histone ubiquitin ligase activity / (E3-independent) E2 ubiquitin-conjugating enzyme / protein K6-linked ubiquitination / Signaling by BMP / double-strand break repair via classical nonhomologous end joining /  isotype switching / protein K11-linked ubiquitination / DNA repair-dependent chromatin remodeling / positive regulation of protein targeting to mitochondrion / K63-linked polyubiquitin modification-dependent protein binding / isotype switching / protein K11-linked ubiquitination / DNA repair-dependent chromatin remodeling / positive regulation of protein targeting to mitochondrion / K63-linked polyubiquitin modification-dependent protein binding /  E2 ubiquitin-conjugating enzyme / response to ionizing radiation / negative regulation of transcription elongation by RNA polymerase II / protein monoubiquitination / protein K63-linked ubiquitination / ubiquitin conjugating enzyme activity / heterochromatin organization / negative regulation of BMP signaling pathway / nucleosomal DNA binding / negative regulation of tumor necrosis factor-mediated signaling pathway / E2 ubiquitin-conjugating enzyme / response to ionizing radiation / negative regulation of transcription elongation by RNA polymerase II / protein monoubiquitination / protein K63-linked ubiquitination / ubiquitin conjugating enzyme activity / heterochromatin organization / negative regulation of BMP signaling pathway / nucleosomal DNA binding / negative regulation of tumor necrosis factor-mediated signaling pathway /  nucleosome binding / negative regulation of megakaryocyte differentiation / protein localization to CENP-A containing chromatin / protein autoubiquitination / protein K48-linked ubiquitination / Chromatin modifying enzymes / Replacement of protamines by nucleosomes in the male pronucleus / interstrand cross-link repair / CENP-A containing nucleosome / SUMOylation of DNA damage response and repair proteins / epigenetic regulation of gene expression / Packaging Of Telomere Ends / nucleosome binding / negative regulation of megakaryocyte differentiation / protein localization to CENP-A containing chromatin / protein autoubiquitination / protein K48-linked ubiquitination / Chromatin modifying enzymes / Replacement of protamines by nucleosomes in the male pronucleus / interstrand cross-link repair / CENP-A containing nucleosome / SUMOylation of DNA damage response and repair proteins / epigenetic regulation of gene expression / Packaging Of Telomere Ends /  ubiquitin ligase complex / Recognition and association of DNA glycosylase with site containing an affected purine / Cleavage of the damaged purine / Deposition of new CENPA-containing nucleosomes at the centromere / Recognition and association of DNA glycosylase with site containing an affected pyrimidine / Cleavage of the damaged pyrimidine / Inhibition of DNA recombination at telomere / Meiotic synapsis / positive regulation of DNA repair / telomere organization / RNA Polymerase I Promoter Opening / Interleukin-7 signaling / Assembly of the ORC complex at the origin of replication / SUMOylation of chromatin organization proteins / TICAM1, RIP1-mediated IKK complex recruitment / ubiquitin ligase complex / Recognition and association of DNA glycosylase with site containing an affected purine / Cleavage of the damaged purine / Deposition of new CENPA-containing nucleosomes at the centromere / Recognition and association of DNA glycosylase with site containing an affected pyrimidine / Cleavage of the damaged pyrimidine / Inhibition of DNA recombination at telomere / Meiotic synapsis / positive regulation of DNA repair / telomere organization / RNA Polymerase I Promoter Opening / Interleukin-7 signaling / Assembly of the ORC complex at the origin of replication / SUMOylation of chromatin organization proteins / TICAM1, RIP1-mediated IKK complex recruitment /  DNA methylation / IKK complex recruitment mediated by RIP1 / Condensation of Prophase Chromosomes / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / SIRT1 negatively regulates rRNA expression / Chromatin modifications during the maternal to zygotic transition (MZT) / HCMV Late Events / DNA methylation / IKK complex recruitment mediated by RIP1 / Condensation of Prophase Chromosomes / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / SIRT1 negatively regulates rRNA expression / Chromatin modifications during the maternal to zygotic transition (MZT) / HCMV Late Events /  innate immune response in mucosa / PRC2 methylates histones and DNA / innate immune response in mucosa / PRC2 methylates histones and DNA /  ubiquitin binding / Defective pyroptosis / Negative regulators of DDX58/IFIH1 signaling / HDACs deacetylate histones / ubiquitin binding / Defective pyroptosis / Negative regulators of DDX58/IFIH1 signaling / HDACs deacetylate histones /  Peroxisomal protein import / RNA Polymerase I Promoter Escape / Downregulation of SMAD2/3:SMAD4 transcriptional activity / Peroxisomal protein import / RNA Polymerase I Promoter Escape / Downregulation of SMAD2/3:SMAD4 transcriptional activity /  lipopolysaccharide binding / Regulation of TNFR1 signaling / Nonhomologous End-Joining (NHEJ) / Transcriptional regulation by small RNAs / Formation of the beta-catenin:TCF transactivating complex / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / RING-type E3 ubiquitin transferase / protein modification process / Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 / NoRC negatively regulates rRNA expression / Oxygen-dependent proline hydroxylation of Hypoxia-inducible Factor Alpha / Inactivation of CSF3 (G-CSF) signaling / G2/M DNA damage checkpoint / HDMs demethylate histones / B-WICH complex positively regulates rRNA expression / DNA Damage/Telomere Stress Induced Senescence / Metalloprotease DUBs / PKMTs methylate histone lysines / RMTs methylate histone arginines / lipopolysaccharide binding / Regulation of TNFR1 signaling / Nonhomologous End-Joining (NHEJ) / Transcriptional regulation by small RNAs / Formation of the beta-catenin:TCF transactivating complex / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / RING-type E3 ubiquitin transferase / protein modification process / Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 / NoRC negatively regulates rRNA expression / Oxygen-dependent proline hydroxylation of Hypoxia-inducible Factor Alpha / Inactivation of CSF3 (G-CSF) signaling / G2/M DNA damage checkpoint / HDMs demethylate histones / B-WICH complex positively regulates rRNA expression / DNA Damage/Telomere Stress Induced Senescence / Metalloprotease DUBs / PKMTs methylate histone lysines / RMTs methylate histone arginines /  Meiotic recombination / Pre-NOTCH Transcription and Translation / Activation of anterior HOX genes in hindbrain development during early embryogenesis / HCMV Early Events / double-strand break repair via nonhomologous end joining / protein polyubiquitination / Transcriptional regulation of granulopoiesis / structural constituent of chromatin / ubiquitin-protein transferase activity / UCH proteinases / Meiotic recombination / Pre-NOTCH Transcription and Translation / Activation of anterior HOX genes in hindbrain development during early embryogenesis / HCMV Early Events / double-strand break repair via nonhomologous end joining / protein polyubiquitination / Transcriptional regulation of granulopoiesis / structural constituent of chromatin / ubiquitin-protein transferase activity / UCH proteinases /  ubiquitin protein ligase activity / double-strand break repair / ubiquitin protein ligase activity / double-strand break repair /  nucleosome / antimicrobial humoral immune response mediated by antimicrobial peptide / nucleosome / antimicrobial humoral immune response mediated by antimicrobial peptide /  nucleosome assembly / Antigen processing: Ubiquitination & Proteasome degradation / E3 ubiquitin ligases ubiquitinate target proteins nucleosome assembly / Antigen processing: Ubiquitination & Proteasome degradation / E3 ubiquitin ligases ubiquitinate target proteinsSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.2 Å cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Hu Q / Botuyan MV / Zhao D / Cui G / Mer G | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2024 Journal: Mol Cell / Year: 2024Title: Mechanisms of RNF168 nucleosome recognition and ubiquitylation. Authors: Qi Hu / Debiao Zhao / Gaofeng Cui / Janarjan Bhandari / James R Thompson / Maria Victoria Botuyan / Georges Mer /  Abstract: RNF168 plays a central role in the DNA damage response (DDR) by ubiquitylating histone H2A at K13 and K15. These modifications direct BRCA1-BARD1 and 53BP1 foci formation in chromatin, essential for ...RNF168 plays a central role in the DNA damage response (DDR) by ubiquitylating histone H2A at K13 and K15. These modifications direct BRCA1-BARD1 and 53BP1 foci formation in chromatin, essential for cell-cycle-dependent DNA double-strand break (DSB) repair pathway selection. The mechanism by which RNF168 catalyzes the targeted accumulation of H2A ubiquitin conjugates to form repair foci around DSBs remains unclear. Here, using cryoelectron microscopy (cryo-EM), nuclear magnetic resonance (NMR) spectroscopy, and functional assays, we provide a molecular description of the reaction cycle and dynamics of RNF168 as it modifies the nucleosome and recognizes its ubiquitylation products. We demonstrate an interaction of a canonical ubiquitin-binding domain within full-length RNF168, which not only engages ubiquitin but also the nucleosome surface, clarifying how such site-specific ubiquitin recognition propels a signal amplification loop. Beyond offering mechanistic insights into a key DDR protein, our study aids in understanding site specificity in both generating and interpreting chromatin ubiquitylation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40606.map.gz emd_40606.map.gz | 59.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40606-v30.xml emd-40606-v30.xml emd-40606.xml emd-40606.xml | 29.7 KB 29.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_40606.png emd_40606.png | 151.9 KB | ||
| Masks |  emd_40606_msk_1.map emd_40606_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-40606.cif.gz emd-40606.cif.gz | 7.6 KB | ||
| Others |  emd_40606_additional_1.map.gz emd_40606_additional_1.map.gz emd_40606_half_map_1.map.gz emd_40606_half_map_1.map.gz emd_40606_half_map_2.map.gz emd_40606_half_map_2.map.gz | 32.5 MB 59.3 MB 59.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40606 http://ftp.pdbj.org/pub/emdb/structures/EMD-40606 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40606 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40606 | HTTPS FTP |
-Related structure data
| Related structure data |  8smyMC  8smwC  8smxC  8smzC  8sn0C  8sn1C  8sn2C  8sn3C  8sn4C  8sn5C  8sn6C  8sn7C  8sn8C  8sn9C  8snaC  8txvC  8txwC  8txxC  8u13C  8u14C  8upfC  8uq8C  8uq9C  8uqaC  8uqbC  8uqcC  8uqdC  8uqeC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_40606.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40606.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.328 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_40606_msk_1.map emd_40606_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: #1
| File | emd_40606_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_40606_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_40606_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Human nucleosome core particle in complex with RNF168 and UbcH5c~Ub
+Supramolecule #1: Human nucleosome core particle in complex with RNF168 and UbcH5c~Ub
+Macromolecule #1: Histone H3.1
+Macromolecule #2: Histone H4
+Macromolecule #3: Histone H2A type 1-B/E
+Macromolecule #4: Histone H2B type 1-J
+Macromolecule #7: E3 ubiquitin-protein ligase RNF168
+Macromolecule #8: Ubiquitin-conjugating enzyme E2 D3
+Macromolecule #5: DNA (147-MER)
+Macromolecule #6: DNA (147-MER)
+Macromolecule #9: ZINC ION
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.4 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
| Details | 10 mM HEPES, 100 mM NaCl, 1 mM DTT, pH 7.5 |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 130000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 14179 / Average electron dose: 60.0 e/Å2 Details: 14179 images were recorded in movie-mode of which 14113 were retained for particle picking. |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Particle selection | Number selected: 16063830 |
|---|---|
| Startup model | Type of model: NONE |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: cryoSPARC (ver. 4.12) |
| Final 3D classification | Software - Name: cryoSPARC (ver. 4.12) |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: cryoSPARC (ver. 4.12) |
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Algorithm: FOURIER SPACE / Resolution.type: BY AUTHOR / Resolution: 3.2 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC (ver. 4.12) / Number images used: 117759 |
-Atomic model buiding 1
| Initial model |
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT | ||||||||
| Output model |  PDB-8smy: |
 Movie
Movie Controller
Controller


































 Z
Z Y
Y X
X





































