[English] 日本語
 Yorodumi
Yorodumi- EMDB-40503: Human GABAA receptor alpha1-beta2-gamma2 subtype in complex with ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human GABAA receptor alpha1-beta2-gamma2 subtype in complex with GABA plus allopregnanolone | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  Ion channel / neurosteroids / Ion channel / neurosteroids /  TRANSPORT PROTEIN TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology information benzodiazepine receptor activity / benzodiazepine receptor activity /  GABA receptor complex / inhibitory extracellular ligand-gated monoatomic ion channel activity / GABA receptor activation / inner ear receptor cell development / GABA-gated chloride ion channel activity / cellular response to histamine / GABA receptor complex / inhibitory extracellular ligand-gated monoatomic ion channel activity / GABA receptor activation / inner ear receptor cell development / GABA-gated chloride ion channel activity / cellular response to histamine /  inhibitory synapse assembly / inhibitory synapse assembly /  GABA-A receptor activity / GABA-A receptor activity /  GABA-A receptor complex ... GABA-A receptor complex ... benzodiazepine receptor activity / benzodiazepine receptor activity /  GABA receptor complex / inhibitory extracellular ligand-gated monoatomic ion channel activity / GABA receptor activation / inner ear receptor cell development / GABA-gated chloride ion channel activity / cellular response to histamine / GABA receptor complex / inhibitory extracellular ligand-gated monoatomic ion channel activity / GABA receptor activation / inner ear receptor cell development / GABA-gated chloride ion channel activity / cellular response to histamine /  inhibitory synapse assembly / inhibitory synapse assembly /  GABA-A receptor activity / GABA-A receptor activity /  GABA-A receptor complex / GABA-A receptor complex /  innervation / postsynaptic specialization membrane / innervation / postsynaptic specialization membrane /  neurotransmitter receptor activity / neurotransmitter receptor activity /  synaptic transmission, GABAergic / gamma-aminobutyric acid signaling pathway / synaptic transmission, GABAergic / gamma-aminobutyric acid signaling pathway /  chloride channel activity / chloride channel activity /  adult behavior / cochlea development / Signaling by ERBB4 / adult behavior / cochlea development / Signaling by ERBB4 /  chloride channel complex / GABA-ergic synapse / regulation of postsynaptic membrane potential / chloride transmembrane transport / dendrite membrane / post-embryonic development / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / cytoplasmic vesicle membrane / chemical synaptic transmission / postsynapse / chloride channel complex / GABA-ergic synapse / regulation of postsynaptic membrane potential / chloride transmembrane transport / dendrite membrane / post-embryonic development / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / cytoplasmic vesicle membrane / chemical synaptic transmission / postsynapse /  postsynaptic membrane / neuron projection / postsynaptic membrane / neuron projection /  axon / axon /  synapse / extracellular exosome / synapse / extracellular exosome /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) / Homo sapiens (human) /   Mus musculus (house mouse) Mus musculus (house mouse) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.98 Å cryo EM / Resolution: 2.98 Å | |||||||||
 Authors Authors | Legesse DH / Hibbs RE | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural insights into opposing actions of neurosteroids on GABA receptors. Authors: Dagimhiwat H Legesse / Chen Fan / Jinfeng Teng / Yuxuan Zhuang / Rebecca J Howard / Colleen M Noviello / Erik Lindahl / Ryan E Hibbs /   Abstract: γ-Aminobutyric acid type A (GABA) receptors mediate fast inhibitory signaling in the brain and are targets of numerous drugs and endogenous neurosteroids. A subset of neurosteroids are GABA receptor ...γ-Aminobutyric acid type A (GABA) receptors mediate fast inhibitory signaling in the brain and are targets of numerous drugs and endogenous neurosteroids. A subset of neurosteroids are GABA receptor positive allosteric modulators; one of these, allopregnanolone, is the only drug approved specifically for treating postpartum depression. There is a consensus emerging from structural, physiological and photolabeling studies as to where positive modulators bind, but how they potentiate GABA activation remains unclear. Other neurosteroids are negative modulators of GABA receptors, but their binding sites remain debated. Here we present structures of a synaptic GABA receptor bound to allopregnanolone and two inhibitory sulfated neurosteroids. Allopregnanolone binds at the receptor-bilayer interface, in the consensus potentiator site. In contrast, inhibitory neurosteroids bind in the pore. MD simulations and electrophysiology support a mechanism by which allopregnanolone potentiates channel activity and suggest the dominant mechanism for sulfated neurosteroid inhibition is through pore block. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40503.map.gz emd_40503.map.gz | 6.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40503-v30.xml emd-40503-v30.xml emd-40503.xml emd-40503.xml | 20.6 KB 20.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_40503.png emd_40503.png | 57.2 KB | ||
| Filedesc metadata |  emd-40503.cif.gz emd-40503.cif.gz | 7 KB | ||
| Others |  emd_40503_half_map_1.map.gz emd_40503_half_map_1.map.gz emd_40503_half_map_2.map.gz emd_40503_half_map_2.map.gz | 71.2 MB 71.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40503 http://ftp.pdbj.org/pub/emdb/structures/EMD-40503 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40503 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40503 | HTTPS FTP |
-Related structure data
| Related structure data |  8si9MC  8sgoC  8sidC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_40503.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40503.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_40503_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_40503_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human GABA-A receptor alpha1-beta2-gamma2 subtype in complex with...
| Entire | Name: Human GABA-A receptor alpha1-beta2-gamma2 subtype in complex with GABA and allopregnanolone |
|---|---|
| Components |
|
-Supramolecule #1: Human GABA-A receptor alpha1-beta2-gamma2 subtype in complex with...
| Supramolecule | Name: Human GABA-A receptor alpha1-beta2-gamma2 subtype in complex with GABA and allopregnanolone type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 / Details: Human GABA-A receptor alpha1-beta2-gamma2 subtype |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Gamma-aminobutyric acid receptor subunit beta-2
| Macromolecule | Name: Gamma-aminobutyric acid receptor subunit beta-2 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 41.810086 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QSVNDPSNMS LVKETVDRLL KGYDIRLRPD FGGPPVAVGM NIDIASIDMV SEVNMDYTLT MYFQQAWRDK RLSYNVIPLN LTLDNRVAD QLWVPDTYFL NDKKSFVHGV TVKNRMIRLH PDGTVLYGLR ITTTAACMMD LRRYPLDEQN CTLEIESYGY T TDDIEFYW ...String: QSVNDPSNMS LVKETVDRLL KGYDIRLRPD FGGPPVAVGM NIDIASIDMV SEVNMDYTLT MYFQQAWRDK RLSYNVIPLN LTLDNRVAD QLWVPDTYFL NDKKSFVHGV TVKNRMIRLH PDGTVLYGLR ITTTAACMMD LRRYPLDEQN CTLEIESYGY T TDDIEFYW RGDDNAVTGV TKIELPQFSI VDYKLITKKV VFSTGSYPRL SLSFKLKRNI GYFILQTYMP SILITILSWV SF WINYDAS AARVALGITT VLTMTTINTH LRETLPKIPY VKAIDMYLMG CFVFVFMALL EYALVNYIFF SQPARAAAID RWS RIFFPV VFSFFNIVYW LYYVNVDGSG ATNFSLLKQA GDVEENPG UniProtKB: Gamma-aminobutyric acid receptor subunit beta-2, Gamma-aminobutyric acid receptor subunit beta-2 |
-Macromolecule #2: Gamma-aminobutyric acid receptor subunit alpha-1
| Macromolecule | Name: Gamma-aminobutyric acid receptor subunit alpha-1 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 41.061211 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QPSLQDELKD NTTVFTRILD RLLDGYDNRL RPGLGERVTE VKTDIFVTSF GPVSDHDMEY TIDVFFRQSW KDERLKFKGP MTVLRLNNL MASKIWTPDT FFHNGKKSVA HNMTMPNKLL RITEDGTLLY TMRLTVRAEC PMHLEDFPMD AHACPLKFGS Y AYTRAEVV ...String: QPSLQDELKD NTTVFTRILD RLLDGYDNRL RPGLGERVTE VKTDIFVTSF GPVSDHDMEY TIDVFFRQSW KDERLKFKGP MTVLRLNNL MASKIWTPDT FFHNGKKSVA HNMTMPNKLL RITEDGTLLY TMRLTVRAEC PMHLEDFPMD AHACPLKFGS Y AYTRAEVV YEWTREPARS VVVAEDGSRL NQYDLLGQTV DSGIVQSSTG EYVVMTTHFH LKRKIGYFVI QTYLPCIMTV IL SQVSFWL NRESVPARTV FGVTTVLTMT TLSISARNSL PKVAYATAMD WFIAVCYAFV FSALIEFATV NYFTKSQPAR AAK IDRLSR IAFPLLFGIF NLVYWATYLN REPQLKAPTP HQ UniProtKB: Gamma-aminobutyric acid receptor subunit alpha-1, Gamma-aminobutyric acid receptor subunit alpha-1 |
-Macromolecule #3: Gamma-aminobutyric acid receptor subunit gamma-2
| Macromolecule | Name: Gamma-aminobutyric acid receptor subunit gamma-2 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 47.673109 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: WSHPQFEKGG GSGGGSGGSS AWSHPQFEKL EVLFQGPQKS DDDYEDYASN KTWVLTPKVP EGDVTVILNN LLEGYDNKLR PDIGVKPTL IHTDMYVNSI GPVNAINMEY TIDIFFAQTW YDRRLKFNST IKVLRLNSNM VGKIWIPDTF FRNSKKADAH W ITTPNRML ...String: WSHPQFEKGG GSGGGSGGSS AWSHPQFEKL EVLFQGPQKS DDDYEDYASN KTWVLTPKVP EGDVTVILNN LLEGYDNKLR PDIGVKPTL IHTDMYVNSI GPVNAINMEY TIDIFFAQTW YDRRLKFNST IKVLRLNSNM VGKIWIPDTF FRNSKKADAH W ITTPNRML RIWNDGRVLY TLRLTIDAEC QLQLHNFPMD EHSCPLEFSS YGYPREEIVY QWKRSSVEVG DTRSWRLYQF SF VGLRNTT EVVKTTSGDY VVMSVYFDLS RRMGYFTIQT YIPCTLIVVL SWVSFWINKD AVPARTSLGI TTVLTMTTLS TIA RKSLPK VSYVTAMDLF VSVCFIFVFS ALVEYGTLHY FVSSQPARAA KMDSYARIFF PTAFCLFNLV YWVSYLYLSR GSGA TNFSL LKQAGDVEEN PG UniProtKB: Gamma-aminobutyric acid receptor subunit gamma-2, Gamma-aminobutyric acid receptor subunit gamma-2 |
-Macromolecule #4: Kappa Fab Light Chain
| Macromolecule | Name: Kappa Fab Light Chain / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Mus musculus (house mouse) Mus musculus (house mouse) |
| Molecular weight | Theoretical: 23.505943 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: NIVMTQSPKS MSMSVGERVT LSCKASEYVG TYVSWYQQKP EQSPKLLIYG ASNRYTGVPD RFTGSGSATD FTLTIGSVQA EDLADYHCG QSYSYPTFGA GTKLELKRAD AAPTVSIFPP SSEQLTSGGA SVVCFLNNFY PKDINVKWKI DGSERQNGVL N SWTDQDSK ...String: NIVMTQSPKS MSMSVGERVT LSCKASEYVG TYVSWYQQKP EQSPKLLIYG ASNRYTGVPD RFTGSGSATD FTLTIGSVQA EDLADYHCG QSYSYPTFGA GTKLELKRAD AAPTVSIFPP SSEQLTSGGA SVVCFLNNFY PKDINVKWKI DGSERQNGVL N SWTDQDSK DSTYSMSSTL TLTKDEYERH NSYTCEATHK TSTSPIVKSF NRNEC |
-Macromolecule #5: IgG2b Fab Heavy Chain
| Macromolecule | Name: IgG2b Fab Heavy Chain / type: protein_or_peptide / ID: 5 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Mus musculus (house mouse) Mus musculus (house mouse) |
| Molecular weight | Theoretical: 49.811043 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: EVQLQQSGAE LVKPGASVKL SCTASGFNIK DTYMYWVKQR PEQGLEWIGR IDPANGDTKY DPKFQGKATI TTDTFSNTAY LQLSSLTSE DTAVYYCARK GLRWAMDYWG QGTSVTVSTA KTTPPSVYPL APGCGDTTGS SVTLGCLVKG YFPESVTVTW N SGSLSSSV ...String: EVQLQQSGAE LVKPGASVKL SCTASGFNIK DTYMYWVKQR PEQGLEWIGR IDPANGDTKY DPKFQGKATI TTDTFSNTAY LQLSSLTSE DTAVYYCARK GLRWAMDYWG QGTSVTVSTA KTTPPSVYPL APGCGDTTGS SVTLGCLVKG YFPESVTVTW N SGSLSSSV HTFPALLQSG LYTMSSSVTV PSSTWPSQTV TCSVAHPASS TTVDKKLEPS GPISTINPCP PCKECHKCPA PN LEGGPSV FIFPPNIKDV LMISLTPKVT CVVVDVSEDD PDVQISWFVN NVEVHTAQTQ THREDYNSTI RVVSTLPIQH QDW MSGKEF KCKVNNKDLP SPIERTISKI KGLVRAPQVY ILPPPAEQLS RKDVSLTCLV VGFNPGDISV EWTSNGHTEE NYKD TAPVL DSDGSYFIYS KLNMKTSKWE KTDSFSCNVR HEGLKNYYLK KTISRSPGK |
-Macromolecule #9: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 9 / Number of copies: 2 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #10: allopregnanolone
| Macromolecule | Name: allopregnanolone / type: ligand / ID: 10 / Number of copies: 2 / Formula: Y4B |
|---|---|
| Molecular weight | Theoretical: 318.493 Da |
| Chemical component information | 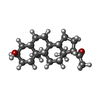 ChemComp-Y4B: |
-Macromolecule #11: GAMMA-AMINO-BUTANOIC ACID
| Macromolecule | Name: GAMMA-AMINO-BUTANOIC ACID / type: ligand / ID: 11 / Number of copies: 2 / Formula: ABU |
|---|---|
| Molecular weight | Theoretical: 103.12 Da |
| Chemical component information |  ChemComp-ABU: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 20.0 µm / Nominal defocus min: 8.0 µm Bright-field microscopy / Nominal defocus max: 20.0 µm / Nominal defocus min: 8.0 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 1.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.98 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 167534 |
 Movie
Movie Controller
Controller








 Z
Z Y
Y X
X


















