+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of bacterioferritin holoform 1a | |||||||||
 Map data Map data | Final Map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  Bacterioferritin / METAL BINDING PROTEIN Bacterioferritin / METAL BINDING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology information ferroxidase / intracellular sequestering of iron ion / ferroxidase / intracellular sequestering of iron ion /  ferroxidase activity / ferroxidase activity /  ferric iron binding / iron ion transport / iron ion binding / ferric iron binding / iron ion transport / iron ion binding /  heme binding / heme binding /  cytosol cytosolSimilarity search - Function | |||||||||
| Biological species |   Streptomyces coelicolor (bacteria) Streptomyces coelicolor (bacteria) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.6 Å cryo EM / Resolution: 3.6 Å | |||||||||
 Authors Authors | Jobichen C / Sivaraman J | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: PNAS Nexus / Year: 2023 Journal: PNAS Nexus / Year: 2023Title: Bacterioferritin nanocage structures uncover the biomineralization process in ferritins. Authors: Chacko Jobichen / Tan Ying Chong / Rajesh Rattinam / Sandip Basak / Mahalashmi Srinivasan / Yeu Khai Choong / Kannu Priya Pandey / Tran Bich Ngoc / Jian Shi / Jayaraman Angayarkanni / J Sivaraman /    Abstract: Iron is an essential element involved in various metabolic processes. The ferritin family of proteins forms nanocage assembly and is involved in iron oxidation, storage, and mineralization. Although ...Iron is an essential element involved in various metabolic processes. The ferritin family of proteins forms nanocage assembly and is involved in iron oxidation, storage, and mineralization. Although several structures of human ferritins and bacterioferritins have been solved, there is still no complete structure that shows both the trapped Fe-biomineral cluster and the nanocage. Furthermore, whereas the mechanism of iron trafficking has been explained using various approaches, structural details on the biomineralization process (i.e. the formation of the mineral itself) are generally lacking. Here, we report the cryo-electron microscopy (cryo-EM) structures of apoform and biomineral bound form (holoforms) of the bacterioferritin (ScBfr) nanocage and the subunit crystal structure. The holoforms show different stages of Fe-biomineral accumulation inside the nanocage, in which the connections exist in two of the fourfold channels of the nanocage between the C-terminal of the ScBfr monomers and the Fe-biomineral cluster. The mutation and truncation of the bacterioferritin residues involved in these connections significantly reduced the iron and phosphate binding in comparison with those of the wild type and together explain the underlying mechanism. Collectively, our results represent a prototype for the bacterioferritin nanocage, which reveals insight into its biomineralization and the potential channel for bacterioferritin-associated iron trafficking. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33640.map.gz emd_33640.map.gz | 57.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33640-v30.xml emd-33640-v30.xml emd-33640.xml emd-33640.xml | 14.8 KB 14.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33640.png emd_33640.png | 244.8 KB | ||
| Others |  emd_33640_half_map_1.map.gz emd_33640_half_map_1.map.gz emd_33640_half_map_2.map.gz emd_33640_half_map_2.map.gz | 46 MB 46 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33640 http://ftp.pdbj.org/pub/emdb/structures/EMD-33640 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33640 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33640 | HTTPS FTP |
-Related structure data
| Related structure data |  7y6gMC  7y6fC  7y6pC  8jaxC  8jb0C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33640.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33640.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Final Map | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.858 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half Map 2
| File | emd_33640_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half Map 1
| File | emd_33640_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Bacterioferritin
| Entire | Name: Bacterioferritin |
|---|---|
| Components |
|
-Supramolecule #1: Bacterioferritin
| Supramolecule | Name: Bacterioferritin / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Streptomyces coelicolor (bacteria) Streptomyces coelicolor (bacteria) |
| Molecular weight | Theoretical: 480 KDa |
-Macromolecule #1: Bacterioferritin
| Macromolecule | Name: Bacterioferritin / type: protein_or_peptide / ID: 1 / Number of copies: 24 / Enantiomer: LEVO / EC number:  ferroxidase ferroxidase |
|---|---|
| Source (natural) | Organism:   Streptomyces coelicolor (bacteria) / Strain: ATCC BAA-471 / A3(2) / M145 Streptomyces coelicolor (bacteria) / Strain: ATCC BAA-471 / A3(2) / M145 |
| Molecular weight | Theoretical: 18.434775 KDa |
| Recombinant expression | Organism:   Escherichia coli 'BL21-Gold(DE3)pLysS AG' (bacteria) Escherichia coli 'BL21-Gold(DE3)pLysS AG' (bacteria) |
| Sequence | String: MQGDPEVIEF LNEQLTAELT AINQYFLHAK LQDHKGWTKL AKYTRAESFD EMRHAEVLTD RILLLDGLPN YQRLFHVRVG QSVTEMFQA DREVELEAID RLRRGIEVMR AKHDITSANV FEAILADEEH HIDYLETQLD LIEKLGESLY LSTVIEQTQ UniProtKB:  Bacterioferritin Bacterioferritin |
-Macromolecule #2: FE (II) ION
| Macromolecule | Name: FE (II) ION / type: ligand / ID: 2 / Number of copies: 24 / Formula: FE2 |
|---|---|
| Molecular weight | Theoretical: 55.845 Da |
-Macromolecule #3: FE (III) ION
| Macromolecule | Name: FE (III) ION / type: ligand / ID: 3 / Number of copies: 23 / Formula: FE |
|---|---|
| Molecular weight | Theoretical: 55.845 Da |
-Macromolecule #4: PROTOPORPHYRIN IX CONTAINING FE
| Macromolecule | Name: PROTOPORPHYRIN IX CONTAINING FE / type: ligand / ID: 4 / Number of copies: 12 / Formula: HEM |
|---|---|
| Molecular weight | Theoretical: 616.487 Da |
| Chemical component information | 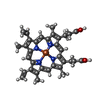 ChemComp-HEM: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.8 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.7000000000000001 µm Bright-field microscopy / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.7000000000000001 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 1.8 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Initial angle assignment | Type: PROJECTION MATCHING |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.6 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 5200 |
 Movie
Movie Controller
Controller












 Z
Z Y
Y X
X


















