+ Open data
Open data
- Basic information
Basic information
| Entry | 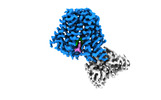 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of hSLC19A1+3'3'-CDA | ||||||||||||||||||
 Map data Map data | |||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationfolic acid transmembrane transporter activity / folate:monoatomic anion antiporter activity / methotrexate transport / folate transmembrane transport / methotrexate transmembrane transporter activity / folic acid transport / folate import across plasma membrane / cyclic-GMP-AMP transmembrane transporter activity / cyclic-GMP-AMP transmembrane import across plasma membrane / organic anion transport ...folic acid transmembrane transporter activity / folate:monoatomic anion antiporter activity / methotrexate transport / folate transmembrane transport / methotrexate transmembrane transporter activity / folic acid transport / folate import across plasma membrane / cyclic-GMP-AMP transmembrane transporter activity / cyclic-GMP-AMP transmembrane import across plasma membrane / organic anion transport / 2',3'-cyclic GMP-AMP binding / xenobiotic transmembrane transport / organic anion transmembrane transporter activity / Metabolism of folate and pterines /  antiporter activity / antiporter activity /  folic acid binding / folic acid metabolic process / xenobiotic transmembrane transporter activity / transport across blood-brain barrier / female pregnancy / brush border membrane / basolateral plasma membrane / apical plasma membrane / folic acid binding / folic acid metabolic process / xenobiotic transmembrane transporter activity / transport across blood-brain barrier / female pregnancy / brush border membrane / basolateral plasma membrane / apical plasma membrane /  plasma membrane plasma membraneSimilarity search - Function | ||||||||||||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.0 Å cryo EM / Resolution: 3.0 Å | ||||||||||||||||||
 Authors Authors | Zhang QX / Zhang XY / Zhu YL / Sun PP / Gao A / Zhang LG / Gao P | ||||||||||||||||||
| Funding support |  China, 5 items China, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Recognition of cyclic dinucleotides and folates by human SLC19A1. Authors: Qixiang Zhang / Xuyuan Zhang / Yalan Zhu / Panpan Sun / Liwei Zhang / Junxiao Ma / Yong Zhang / Lingan Zeng / Xiaohua Nie / Yina Gao / Zhaolong Li / Songqing Liu / Jizhong Lou / Ang Gao / Liguo Zhang / Pu Gao /  Abstract: Cyclic dinucleotides (CDNs) are ubiquitous signalling molecules in all domains of life. Mammalian cells produce one CDN, 2'3'-cGAMP, through cyclic GMP-AMP synthase after detecting cytosolic DNA ...Cyclic dinucleotides (CDNs) are ubiquitous signalling molecules in all domains of life. Mammalian cells produce one CDN, 2'3'-cGAMP, through cyclic GMP-AMP synthase after detecting cytosolic DNA signals. 2'3'-cGAMP, as well as bacterial and synthetic CDN analogues, can act as second messengers to activate stimulator of interferon genes (STING) and elicit broad downstream responses. Extracellular CDNs must traverse the cell membrane to activate STING, a process that is dependent on the solute carrier SLC19A1. Moreover, SLC19A1 represents the major transporter for folate nutrients and antifolate therapeutics, thereby placing SLC19A1 as a key factor in multiple physiological and pathological processes. How SLC19A1 recognizes and transports CDNs, folate and antifolate is unclear. Here we report cryo-electron microscopy structures of human SLC19A1 (hSLC19A1) in a substrate-free state and in complexes with multiple CDNs from different sources, a predominant natural folate and a new-generation antifolate drug. The structural and mutagenesis results demonstrate that hSLC19A1 uses unique yet divergent mechanisms to recognize CDN- and folate-type substrates. Two CDN molecules bind within the hSLC19A1 cavity as a compact dual-molecule unit, whereas folate and antifolate bind as a monomer and occupy a distinct pocket of the cavity. Moreover, the structures enable accurate mapping and potential mechanistic interpretation of hSLC19A1 with loss-of-activity and disease-related mutations. Our research provides a framework for understanding the mechanism of SLC19-family transporters and is a foundation for the development of potential therapeutics. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33387.map.gz emd_33387.map.gz | 116.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33387-v30.xml emd-33387-v30.xml emd-33387.xml emd-33387.xml | 15.5 KB 15.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33387.png emd_33387.png | 46.6 KB | ||
| Others |  emd_33387_half_map_1.map.gz emd_33387_half_map_1.map.gz emd_33387_half_map_2.map.gz emd_33387_half_map_2.map.gz | 98.6 MB 98.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33387 http://ftp.pdbj.org/pub/emdb/structures/EMD-33387 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33387 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33387 | HTTPS FTP |
-Related structure data
| Related structure data |  7xq0MC  7xpzC  7xq1C  7xq2C  8goeC  8gofC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33387.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33387.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_33387_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_33387_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : SLC19A1+3'3'-CDA
| Entire | Name: SLC19A1+3'3'-CDA |
|---|---|
| Components |
|
-Supramolecule #1: SLC19A1+3'3'-CDA
| Supramolecule | Name: SLC19A1+3'3'-CDA / type: complex / Chimera: Yes / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Reduced folate transporter
| Macromolecule | Name: Reduced folate transporter / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 60.556082 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MGSMVPSSPA VEKQVPVEPG PDPELRSWRH LVCYLCFYGF MAQIRPGESF ITPYLLGPDK NFTREQVTNE ITPVLSYSYL AVLVPVFLL TDYLRYTPVL LLQGLSFVSV WLLLLLGHSV AHMQLMELFY SVTMAARIAY SSYIFSLVRP ARYQRVAGYS R AAVLLGVF ...String: MGSMVPSSPA VEKQVPVEPG PDPELRSWRH LVCYLCFYGF MAQIRPGESF ITPYLLGPDK NFTREQVTNE ITPVLSYSYL AVLVPVFLL TDYLRYTPVL LLQGLSFVSV WLLLLLGHSV AHMQLMELFY SVTMAARIAY SSYIFSLVRP ARYQRVAGYS R AAVLLGVF TSSVLGQLLV TVGRVSFSTL NYISLAFLTF SVVLALFLKR PKRSLFFNRD DRGRCETSAS ELERMNPGPG GK LGHALRV ACGDSVLARM LRELGDSLRR PQLRLWSLWW VFNSAGYYLV VYYVHILWNE VDPTTNSARV YNGAADAAST LLG AITSFA AGFVKIRWAR WSKLLIAGVT ATQAGLVFLL AHTRHPSSIW LCYAAFVLFR GSYQFLVPIA TFQIASSLSK ELCA LVFGV NTFFATIVKT IITFIVSDVR GLGLPVRKQF QLYSVYFLIL SIIYFLGAML DGLRHCQRGH HPRQPPAQGL RSAAE EKAA QALSVQDKGL GGLQPAQSPP LSPEDKLGSE NLYLEVLFQG PFQGGSGGSG HHHHHHHHHH |
-Macromolecule #2: (2R,3R,3aS,5R,7aR,9R,10R,10aS,12R,14aR)-2,9-bis(6-amino-9H-purin-...
| Macromolecule | Name: (2R,3R,3aS,5R,7aR,9R,10R,10aS,12R,14aR)-2,9-bis(6-amino-9H-purin-9-yl)octahydro-2H,7H-difuro[3,2-d:3',2'-j][1,3,7,9,2,8 ]tetraoxadiphosphacyclododecine-3,5,10,12-tetrol 5,12-dioxide type: ligand / ID: 2 / Number of copies: 2 / Formula: 2BA |
|---|---|
| Molecular weight | Theoretical: 658.412 Da |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.8 µm Bright-field microscopy / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.8 µm |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Average electron dose: 60.0 e/Å2 |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
- Image processing
Image processing
| Initial angle assignment | Type: PROJECTION MATCHING |
|---|---|
| Final angle assignment | Type: PROJECTION MATCHING |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.0 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 242868 |
 Movie
Movie Controller
Controller






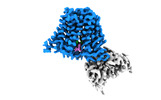






 Z
Z Y
Y X
X

















