[English] 日本語
 Yorodumi
Yorodumi- EMDB-23853: Mouse TRPV3 in MSP2N2 nanodiscs, closed state at 4 degrees Celsius -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23853 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Mouse TRPV3 in MSP2N2 nanodiscs, closed state at 4 degrees Celsius | |||||||||||||||
 Map data Map data | TRPV3 in MSP2N2 nanodiscs, closed state at 4 degrees Celsius | |||||||||||||||
 Sample Sample |
| |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of hair cycle /  TRP channels / response to temperature stimulus / positive regulation of calcium ion import / monoatomic cation channel activity / TRP channels / response to temperature stimulus / positive regulation of calcium ion import / monoatomic cation channel activity /  calcium channel activity / monoatomic ion channel activity / calcium channel activity / monoatomic ion channel activity /  receptor complex / receptor complex /  membrane / identical protein binding membrane / identical protein bindingSimilarity search - Function | |||||||||||||||
| Biological species |   Mus musculus (house mouse) Mus musculus (house mouse) | |||||||||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 1.98 Å cryo EM / Resolution: 1.98 Å | |||||||||||||||
 Authors Authors | Neuberger A / Nadezhdin KD / Sobolevsky AI | |||||||||||||||
| Funding support |  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2021 Journal: Nat Struct Mol Biol / Year: 2021Title: Structural mechanism of heat-induced opening of a temperature-sensitive TRP channel. Authors: Kirill D Nadezhdin / Arthur Neuberger / Yuri A Trofimov / Nikolay A Krylov / Viktor Sinica / Nikita Kupko / Viktorie Vlachova / Eleonora Zakharian / Roman G Efremov / Alexander I Sobolevsky /    Abstract: Numerous physiological functions rely on distinguishing temperature through temperature-sensitive transient receptor potential channels (thermo-TRPs). Although the function of thermo-TRPs has been ...Numerous physiological functions rely on distinguishing temperature through temperature-sensitive transient receptor potential channels (thermo-TRPs). Although the function of thermo-TRPs has been studied extensively, structural determination of their heat- and cold-activated states has remained a challenge. Here, we present cryo-EM structures of the nanodisc-reconstituted wild-type mouse TRPV3 in three distinct conformations: closed, heat-activated sensitized and open states. The heat-induced transformations of TRPV3 are accompanied by changes in the secondary structure of the S2-S3 linker and the N and C termini and represent a conformational wave that links these parts of the protein to a lipid occupying the vanilloid binding site. State-dependent differences in the behavior of bound lipids suggest their active role in thermo-TRP temperature-dependent gating. Our structural data, supported by physiological recordings and molecular dynamics simulations, provide an insight for understanding the molecular mechanism of temperature sensing. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23853.map.gz emd_23853.map.gz | 12.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23853-v30.xml emd-23853-v30.xml emd-23853.xml emd-23853.xml | 16 KB 16 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_23853.png emd_23853.png | 150.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23853 http://ftp.pdbj.org/pub/emdb/structures/EMD-23853 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23853 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23853 | HTTPS FTP |
-Related structure data
| Related structure data |  7mijMC  7mikC  7milC  7mimC  7minC  7mioC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23853.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23853.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | TRPV3 in MSP2N2 nanodiscs, closed state at 4 degrees Celsius | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.86 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Transient receptor potential cation channel subfamily V member 3
| Entire | Name: Transient receptor potential cation channel subfamily V member 3 |
|---|---|
| Components |
|
-Supramolecule #1: Transient receptor potential cation channel subfamily V member 3
| Supramolecule | Name: Transient receptor potential cation channel subfamily V member 3 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Mus musculus (house mouse) Mus musculus (house mouse) |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 924.7 KDa |
-Macromolecule #1: Transient receptor potential cation channel subfamily V member 3
| Macromolecule | Name: Transient receptor potential cation channel subfamily V member 3 type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Mus musculus (house mouse) Mus musculus (house mouse) |
| Molecular weight | Theoretical: 92.630695 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MNAHSKEMAP LMGKRTTAPG GNPVVLTEKR PADLTPTKKS AHFFLEIEGF EPNPTVTKTS PPIFSKPMDS NIRQCLSGNC DDMDSPQSP QDDVTETPSN PNSPSANLAK EEQRQKKKRL KKRIFAAVSE GCVEELRELL QDLQDLCRRR RGLDVPDFLM H KLTASDTG ...String: MNAHSKEMAP LMGKRTTAPG GNPVVLTEKR PADLTPTKKS AHFFLEIEGF EPNPTVTKTS PPIFSKPMDS NIRQCLSGNC DDMDSPQSP QDDVTETPSN PNSPSANLAK EEQRQKKKRL KKRIFAAVSE GCVEELRELL QDLQDLCRRR RGLDVPDFLM H KLTASDTG KTCLMKALLN INPNTKEIVR ILLAFAEEND ILDRFINAEY TEEAYEGQTA LNIAIERRQG DITAVLIAAG AD VNAHAKG VFFNPKYQHE GFYFGETPLA LAACTNQPEI VQLLMENEQT DITSQDSRGN NILHALVTVA EDFKTQNDFV KRM YDMILL RSGNWELETM RNNDGLTPLQ LAAKMGKAEI LKYILSREIK EKPLRSLSRK FTDWAYGPVS SSLYDLTNVD TTTD NSVLE IIVYNTNIDN RHEMLTLEPL HTLLHTKWKK FAKYMFFLSF CFYFFYNITL TLVSYYRPRE DEDLPHPLAL THKMS WLQL LGRMFVLIWA TCISVKEGIA IFLLRPSDLQ SILSDAWFHF VFFVQAVLVI LSVFLYLFAY KEYLACLVLA MALGWA NML YYTRGFQSMG MYSVMIQKVI LHDVLKFLFV YILFLLGFGV ALASLIEKCS KDKKDCSSYG SFSDAVLELF KLTIGLG DL NIQQNSTYPI LFLFLLITYV ILTFVLLLNM LIALMGETVE NVSKESERIW RLQRARTILE FEKMLPEWLR SRFRMGEL C KVADEDFRLC LRINEVKWTE WKTHVSFLNE DPGPIRRTAD LNKIQDSSRS NSKTTLYAFD ELDEFPETSV LVPRGSAAA WSHPQFEK |
-Macromolecule #2: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(tri...
| Macromolecule | Name: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(trimethylammonio)ethyl phosphate type: ligand / ID: 2 / Number of copies: 36 / Formula: POV |
|---|---|
| Molecular weight | Theoretical: 760.076 Da |
| Chemical component information | 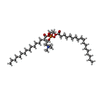 ChemComp-POV: |
-Macromolecule #3: SODIUM ION
| Macromolecule | Name: SODIUM ION / type: ligand / ID: 3 / Number of copies: 2 |
|---|---|
| Molecular weight | Theoretical: 22.99 Da |
-Macromolecule #4: water
| Macromolecule | Name: water / type: ligand / ID: 4 / Number of copies: 328 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.6 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||||||||
| Grid | Model: C-flat-1.2/1.3 / Material: GOLD / Mesh: 200 / Support film - Material: GOLD / Support film - topology: HOLEY | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: -2.0 µm / Nominal defocus min: -0.8 µm Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: -2.0 µm / Nominal defocus min: -0.8 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Number grids imaged: 1 / Number real images: 9702 / Average exposure time: 2.5 sec. / Average electron dose: 58.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Particle selection | Number selected: 4662531 |
|---|---|
| CTF correction | Software - Name: RELION (ver. 3.1) |
| Initial angle assignment | Type: OTHER / Software - Name: RELION (ver. 3.1) |
| Final 3D classification | Software - Name: RELION (ver. 3.1) |
| Final angle assignment | Type: OTHER / Software - Name: RELION (ver. 3.1) |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 1.98 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 3.1) / Number images used: 372121 |
-Atomic model buiding 1
| Refinement | Space: REAL |
|---|---|
| Output model |  PDB-7mij: |
 Movie
Movie Controller
Controller

















