[English] 日本語
 Yorodumi
Yorodumi- EMDB-13233: Cryo-Em structure of the hexameric RUVBL1-RUVBL2 in complex with ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-13233 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-Em structure of the hexameric RUVBL1-RUVBL2 in complex with ZNHIT2 | ||||||||||||
 Map data Map data | RUVBL1-RUVBL2 AAA ring map | ||||||||||||
 Sample Sample |
| ||||||||||||
| Function / homology |  Function and homology information Function and homology informationpromoter-enhancer loop anchoring activity / regulation of DNA strand elongation / positive regulation of telomere maintenance in response to DNA damage / establishment of protein localization to chromatin / R2TP complex / Swr1 complex / dynein axonemal particle / RPAP3/R2TP/prefoldin-like complex / regulation of double-strand break repair / positive regulation of telomerase RNA localization to Cajal body ...promoter-enhancer loop anchoring activity / regulation of DNA strand elongation / positive regulation of telomere maintenance in response to DNA damage / establishment of protein localization to chromatin / R2TP complex / Swr1 complex / dynein axonemal particle / RPAP3/R2TP/prefoldin-like complex / regulation of double-strand break repair / positive regulation of telomerase RNA localization to Cajal body / Ino80 complex / box C/D snoRNP assembly / protein folding chaperone complex /  NuA4 histone acetyltransferase complex / regulation of chromosome organization / positive regulation of double-strand break repair via homologous recombination / NuA4 histone acetyltransferase complex / regulation of chromosome organization / positive regulation of double-strand break repair via homologous recombination /  regulation of DNA replication / regulation of DNA replication /  MLL1 complex / TFIID-class transcription factor complex binding / MLL1 complex / TFIID-class transcription factor complex binding /  regulation of embryonic development / Telomere Extension By Telomerase / RNA polymerase II core promoter sequence-specific DNA binding / regulation of embryonic development / Telomere Extension By Telomerase / RNA polymerase II core promoter sequence-specific DNA binding /  regulation of DNA repair / Deposition of new CENPA-containing nucleosomes at the centromere / positive regulation of DNA repair / TBP-class protein binding / regulation of DNA repair / Deposition of new CENPA-containing nucleosomes at the centromere / positive regulation of DNA repair / TBP-class protein binding /  DNA helicase activity / DNA helicase activity /  telomere maintenance / cellular response to estradiol stimulus / telomere maintenance / cellular response to estradiol stimulus /  ADP binding / Formation of the beta-catenin:TCF transactivating complex / DNA Damage Recognition in GG-NER / ADP binding / Formation of the beta-catenin:TCF transactivating complex / DNA Damage Recognition in GG-NER /  euchromatin / negative regulation of canonical Wnt signaling pathway / chromatin DNA binding / euchromatin / negative regulation of canonical Wnt signaling pathway / chromatin DNA binding /  beta-catenin binding / beta-catenin binding /  nuclear matrix / positive regulation of canonical Wnt signaling pathway / transcription corepressor activity / UCH proteinases / cellular response to UV / unfolded protein binding / nuclear matrix / positive regulation of canonical Wnt signaling pathway / transcription corepressor activity / UCH proteinases / cellular response to UV / unfolded protein binding /  nucleosome / nucleosome /  protein folding / HATs acetylate histones / protein folding / HATs acetylate histones /  ATPase binding / ATPase binding /  spermatogenesis / regulation of apoptotic process / DNA recombination / spermatogenesis / regulation of apoptotic process / DNA recombination /  DNA helicase / DNA helicase /  transcription coactivator activity / protein stabilization / transcription coactivator activity / protein stabilization /  regulation of cell cycle / Ub-specific processing proteases / regulation of cell cycle / Ub-specific processing proteases /  chromatin remodeling / chromatin remodeling /  cadherin binding / cadherin binding /  cell cycle / cell cycle /  ribonucleoprotein complex / RNA polymerase II cis-regulatory region sequence-specific DNA binding / ribonucleoprotein complex / RNA polymerase II cis-regulatory region sequence-specific DNA binding /  cell division / cell division /  DNA repair / DNA repair /  centrosome / regulation of DNA-templated transcription / regulation of transcription by RNA polymerase II / positive regulation of DNA-templated transcription / centrosome / regulation of DNA-templated transcription / regulation of transcription by RNA polymerase II / positive regulation of DNA-templated transcription /  ATP hydrolysis activity / protein homodimerization activity / positive regulation of transcription by RNA polymerase II / extracellular exosome / ATP hydrolysis activity / protein homodimerization activity / positive regulation of transcription by RNA polymerase II / extracellular exosome /  nucleoplasm / nucleoplasm /  ATP binding / ATP binding /  membrane / identical protein binding / membrane / identical protein binding /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 4.1 Å cryo EM / Resolution: 4.1 Å | ||||||||||||
 Authors Authors | Llorca O / Serna M / Fernandez-Leiro R | ||||||||||||
| Funding support |  Spain, 3 items Spain, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2022 Journal: Nucleic Acids Res / Year: 2022Title: CryoEM of RUVBL1-RUVBL2-ZNHIT2, a complex that interacts with pre-mRNA-processing-splicing factor 8. Authors: Marina Serna / Ana González-Corpas / Sofía Cabezudo / Andrés López-Perrote / Gianluca Degliesposti / Eduardo Zarzuela / J Mark Skehel / Javier Muñoz / Oscar Llorca /   Abstract: Biogenesis of the U5 small nuclear ribonucleoprotein (snRNP) is an essential and highly regulated process. In particular, PRPF8, one of U5 snRNP main components, requires HSP90 working in concert ...Biogenesis of the U5 small nuclear ribonucleoprotein (snRNP) is an essential and highly regulated process. In particular, PRPF8, one of U5 snRNP main components, requires HSP90 working in concert with R2TP, a cochaperone complex containing RUVBL1 and RUVBL2 AAA-ATPases, and additional factors that are still poorly characterized. Here, we use biochemistry, interaction mapping, mass spectrometry and cryoEM to study the role of ZNHIT2 in the regulation of the R2TP chaperone during the biogenesis of PRPF8. ZNHIT2 forms a complex with R2TP which depends exclusively on the direct interaction of ZNHIT2 with the RUVBL1-RUVBL2 ATPases. The cryoEM analysis of this complex reveals that ZNHIT2 alters the conformation and nucleotide state of RUVBL1-RUVBL2, affecting its ATPase activity. We characterized the interactions between R2TP, PRPF8, ZNHIT2, ECD and AAR2 proteins. Interestingly, PRPF8 makes a direct interaction with R2TP and this complex can incorporate ZNHIT2 and other proteins involved in the biogenesis of PRPF8 such as ECD and AAR2. Together, these results show that ZNHIT2 participates in the assembly of the U5 snRNP as part of a network of contacts between assembly factors required for PRPF8 biogenesis and the R2TP-HSP90 chaperone, while concomitantly regulating the structure and nucleotide state of R2TP. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13233.map.gz emd_13233.map.gz | 89.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13233-v30.xml emd-13233-v30.xml emd-13233.xml emd-13233.xml | 26.4 KB 26.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_13233.png emd_13233.png | 67.5 KB | ||
| Others |  emd_13233_additional_1.map.gz emd_13233_additional_1.map.gz emd_13233_additional_2.map.gz emd_13233_additional_2.map.gz emd_13233_additional_3.map.gz emd_13233_additional_3.map.gz emd_13233_additional_4.map.gz emd_13233_additional_4.map.gz emd_13233_additional_5.map.gz emd_13233_additional_5.map.gz emd_13233_half_map_1.map.gz emd_13233_half_map_1.map.gz emd_13233_half_map_2.map.gz emd_13233_half_map_2.map.gz | 167.9 MB 140.5 MB 140.7 MB 140.8 MB 166.3 MB 164.9 MB 164.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13233 http://ftp.pdbj.org/pub/emdb/structures/EMD-13233 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13233 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13233 | HTTPS FTP |
-Related structure data
| Related structure data |  7p6xMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_13233.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13233.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RUVBL1-RUVBL2 AAA ring map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.065 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
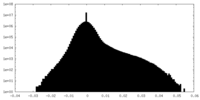
-Additional map: RUVBL1-RUVBL2 AAA ring sharpened map
| File | emd_13233_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RUVBL1-RUVBL2 AAA ring sharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: RUVBL1-RUVBL2-ZNHIT2 consensus map
| File | emd_13233_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RUVBL1-RUVBL2-ZNHIT2 consensus map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: RUVBL-1RUVBL2-ZNHIT2 consensus half map 1
| File | emd_13233_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RUVBL-1RUVBL2-ZNHIT2 consensus half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: RUVBL-1RUVBL2-ZNHIT2 consensus half map 2
| File | emd_13233_additional_4.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RUVBL-1RUVBL2-ZNHIT2 consensus half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: #1
| File | emd_13233_additional_5.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
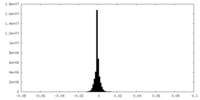
-Half map: RUVBL1-RUVBL2 AAA ring half map 1
| File | emd_13233_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RUVBL1-RUVBL2 AAA ring half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: RUVBL1-RUVBL2 AAA ring half map 2
| File | emd_13233_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RUVBL1-RUVBL2 AAA ring half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Hexameric RUVBL1-RUVBL2 AAA ring in complex with ZNHIT2
| Entire | Name: Hexameric RUVBL1-RUVBL2 AAA ring in complex with ZNHIT2 |
|---|---|
| Components |
|
-Supramolecule #1: Hexameric RUVBL1-RUVBL2 AAA ring in complex with ZNHIT2
| Supramolecule | Name: Hexameric RUVBL1-RUVBL2 AAA ring in complex with ZNHIT2 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: RuvB-like 1
| Macromolecule | Name: RuvB-like 1 / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO / EC number:  DNA helicase DNA helicase |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 50.296914 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MKIEEVKSTT KTQRIASHSH VKGLGLDESG LAKQAASGLV GQENAREACG VIVELIKSKK MAGRAVLLAG PPGTGKTALA LAIAQELGS KVPFCPMVGS EVYSTEIKKT EVLMENFRRA IGLRIKETKE VYEGEVTELT PCETENPMGG YGKTISHVII G LKTAKGTK ...String: MKIEEVKSTT KTQRIASHSH VKGLGLDESG LAKQAASGLV GQENAREACG VIVELIKSKK MAGRAVLLAG PPGTGKTALA LAIAQELGS KVPFCPMVGS EVYSTEIKKT EVLMENFRRA IGLRIKETKE VYEGEVTELT PCETENPMGG YGKTISHVII G LKTAKGTK QLKLDPSIFE SLQKERVEAG DVIYIEANSG AVKRQGRCDT YATEFDLEAE EYVPLPKGDV HKKKEIIQDV TL HDLDVAN ARPQGGQDIL SMMGQLMKPK KTEITDKLRG EINKVVNKYI DQGIAELVPG VLFVDEVHML DIECFTYLHR ALE SSIAPI VIFASNRGNC VIRGTEDITS PHGIPLDLLD RVMIIRTMLY TPQEMKQIIK IRAQTEGINI SEEALNHLGE IGTK TTLRY SVQLLTPANL LAKINGKDSI EKEHVEEISE LFYDAKSSAK ILADQQDKYM K |
-Macromolecule #2: RuvB-like 2
| Macromolecule | Name: RuvB-like 2 / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO / EC number:  DNA helicase DNA helicase |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 51.222465 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MATVTATTKV PEIRDVTRIE RIGAHSHIRG LGLDDALEPR QASQGMVGQL AARRAAGVVL EMIREGKIAG RAVLIAGQPG TGKTAIAMG MAQALGPDTP FTAIAGSEIF SLEMSKTEAL TQAFRRSIGV RIKEETEIIE GEVVEIQIDR PATGTGSKVG K LTLKTTEM ...String: MATVTATTKV PEIRDVTRIE RIGAHSHIRG LGLDDALEPR QASQGMVGQL AARRAAGVVL EMIREGKIAG RAVLIAGQPG TGKTAIAMG MAQALGPDTP FTAIAGSEIF SLEMSKTEAL TQAFRRSIGV RIKEETEIIE GEVVEIQIDR PATGTGSKVG K LTLKTTEM ETIYDLGTKM IESLTKDKVQ AGDVITIDKA TGKISKLGRS FTRARDYDAM GSQTKFVQCP DGELQKRKEV VH TVSLHEI DVINSRTQGF LALFSGDTGE IKSEVREQIN AKVAEWREEG KAEIIPGVLF IDEVHMLDIE SFSFLNRALE SDM APVLIM ATNRGITRIR GTSYQSPHGI PIDLLDRLLI VSTTPYSEKD TKQILRIRCE EEDVEMSEDA YTVLTRIGLE TSLR YAIQL ITAASLVCRK RKGTEVQVDD IKRVYSLFLD ESRSTQYMKE YQDAFLFNEL KGETMDTS |
-Macromolecule #3: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 3 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information | 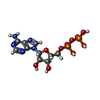 ChemComp-ADP: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy Bright-field microscopy |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 40.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE / Details: Ab initio (Cryosparc) |
|---|---|
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 4.1 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 173905 |
 Movie
Movie Controller
Controller













 Z
Z Y
Y X
X