+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8jta | ||||||
|---|---|---|---|---|---|---|---|
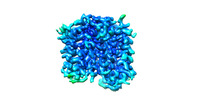
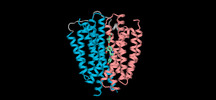
| Title | Human VMAT2 complex with tetrabenazine | ||||||
 Components Components | Synaptic vesicular amine transporter | ||||||
 Keywords Keywords |  TRANSPORT PROTEIN / transporter2 TRANSPORT PROTEIN / transporter2 | ||||||
| Function / homology |  Function and homology information Function and homology informationserotonin secretion by mast cell / sequestering of neurotransmitter / somato-dendritic dopamine secretion / histamine uptake / neurotransmitter loading into synaptic vesicle / monoamine:proton antiporter activity / aminergic neurotransmitter loading into synaptic vesicle / clathrin-sculpted monoamine transport vesicle membrane / Serotonin Neurotransmitter Release Cycle / serotonin:sodium:chloride symporter activity ...serotonin secretion by mast cell / sequestering of neurotransmitter / somato-dendritic dopamine secretion / histamine uptake / neurotransmitter loading into synaptic vesicle / monoamine:proton antiporter activity / aminergic neurotransmitter loading into synaptic vesicle / clathrin-sculpted monoamine transport vesicle membrane / Serotonin Neurotransmitter Release Cycle / serotonin:sodium:chloride symporter activity / Dopamine Neurotransmitter Release Cycle / Norepinephrine Neurotransmitter Release Cycle / serotonin uptake / dopaminergic synapse / dopamine transport / Na+/Cl- dependent neurotransmitter transporters / monoamine transmembrane transporter activity / monoamine transport / histamine secretion by mast cell /  neurotransmitter transport / negative regulation of reactive oxygen species biosynthetic process / response to amphetamine / post-embryonic development / secretory granule membrane / locomotory behavior / neurotransmitter transport / negative regulation of reactive oxygen species biosynthetic process / response to amphetamine / post-embryonic development / secretory granule membrane / locomotory behavior /  terminal bouton / response to toxic substance / synaptic vesicle membrane / terminal bouton / response to toxic substance / synaptic vesicle membrane /  synaptic vesicle / chemical synaptic transmission / synaptic vesicle / chemical synaptic transmission /  axon / intracellular membrane-bounded organelle / axon / intracellular membrane-bounded organelle /  centrosome / centrosome /  dendrite / dendrite /  membrane / membrane /  plasma membrane plasma membraneSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.36 Å cryo EM / Resolution: 3.36 Å | ||||||
 Authors Authors | Jiang, D.H. / Wu, D. | ||||||
| Funding support |  China, 1items China, 1items
| ||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: Transport and inhibition mechanisms of human VMAT2. Authors: Di Wu / Qihao Chen / Zhuoya Yu / Bo Huang / Jun Zhao / Yuhang Wang / Jiawei Su / Feng Zhou / Rui Yan / Na Li / Yan Zhao / Daohua Jiang /  Abstract: Vesicular monoamine transporter 2 (VMAT2) accumulates monoamines in presynaptic vesicles for storage and exocytotic release, and has a vital role in monoaminergic neurotransmission. Dysfunction of ...Vesicular monoamine transporter 2 (VMAT2) accumulates monoamines in presynaptic vesicles for storage and exocytotic release, and has a vital role in monoaminergic neurotransmission. Dysfunction of monoaminergic systems causes many neurological and psychiatric disorders, including Parkinson's disease, hyperkinetic movement disorders and depression. Suppressing VMAT2 with reserpine and tetrabenazine alleviates symptoms of hypertension and Huntington's disease, respectively. Here we describe cryo-electron microscopy structures of human VMAT2 complexed with serotonin and three clinical drugs at 3.5-2.8 Å, demonstrating the structural basis for transport and inhibition. Reserpine and ketanserin occupy the substrate-binding pocket and lock VMAT2 in cytoplasm-facing and lumen-facing states, respectively, whereas tetrabenazine binds in a VMAT2-specific pocket and traps VMAT2 in an occluded state. The structures in three distinct states also reveal the structural basis of the VMAT2 transport cycle. Our study establishes a structural foundation for the mechanistic understanding of substrate recognition, transport, drug inhibition and pharmacology of VMAT2 while shedding light on the rational design of potential therapeutic agents. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8jta.cif.gz 8jta.cif.gz | 77.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8jta.ent.gz pdb8jta.ent.gz | 55.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8jta.json.gz 8jta.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jt/8jta https://data.pdbj.org/pub/pdb/validation_reports/jt/8jta ftp://data.pdbj.org/pub/pdb/validation_reports/jt/8jta ftp://data.pdbj.org/pub/pdb/validation_reports/jt/8jta | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  36638MC  8jswC  8jt9C  8jtcC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 49086.848 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: SLC18A2, SVMT, VMAT2 / Production host: Homo sapiens (human) / Gene: SLC18A2, SVMT, VMAT2 / Production host:   Homo sapiens (human) / References: UniProt: Q05940 Homo sapiens (human) / References: UniProt: Q05940 |
|---|---|
| #2: Chemical | ChemComp-YHL / |
| Has ligand of interest | Y |
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: transporter2 / Type: COMPLEX / Entity ID: #1 / Source: RECOMBINANT |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 8 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2000 nm / Nominal defocus min: 1000 nm Bright-field microscopy / Nominal defocus max: 2000 nm / Nominal defocus min: 1000 nm |
| Image recording | Electron dose: 60 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
3D reconstruction | Resolution: 3.36 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 167230 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller




 PDBj
PDBj






