+Search query
-Structure paper
| Title | High resolution cryo-EM and crystallographic snapshots of the actinobacterial two-in-one 2-oxoglutarate dehydrogenase. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 14, Issue 1, Page 4851, Year 2023 |
| Publish date | Aug 10, 2023 |
 Authors Authors | Lu Yang / Tristan Wagner / Ariel Mechaly / Alexandra Boyko / Eduardo M Bruch / Daniela Megrian / Francesca Gubellini / Pedro M Alzari / Marco Bellinzoni /     |
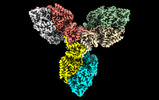
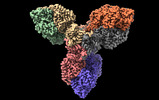
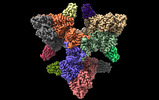
| PubMed Abstract | Actinobacteria possess unique ways to regulate the oxoglutarate metabolic node. Contrary to most organisms in which three enzymes compose the 2-oxoglutarate dehydrogenase complex (ODH), ...Actinobacteria possess unique ways to regulate the oxoglutarate metabolic node. Contrary to most organisms in which three enzymes compose the 2-oxoglutarate dehydrogenase complex (ODH), actinobacteria rely on a two-in-one protein (OdhA) in which both the oxidative decarboxylation and succinyl transferase steps are carried out by the same polypeptide. Here we describe high-resolution cryo-EM and crystallographic snapshots of representative enzymes from Mycobacterium smegmatis and Corynebacterium glutamicum, showing that OdhA is an 800-kDa homohexamer that assembles into a three-blade propeller shape. The obligate trimeric and dimeric states of the acyltransferase and dehydrogenase domains, respectively, are critical for maintaining the overall assembly, where both domains interact via subtle readjustments of their interfaces. Complexes obtained with substrate analogues, reaction products and allosteric regulators illustrate how these domains operate. Furthermore, we provide additional insights into the phosphorylation-dependent regulation of this enzymatic machinery by the signalling protein OdhI. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:37563123 / PubMed:37563123 /  PubMed Central PubMed Central |
| Methods | EM (single particle) / X-ray diffraction |
| Resolution | 2.07 - 4.562 Å |
| Structure data | EMDB-17452, PDB-8p5t: EMDB-17453, PDB-8p5u: EMDB-17454, PDB-8p5v: EMDB-17455, PDB-8p5w: EMDB-17456, PDB-8p5x:  PDB-8p5r:  PDB-8p5s: |
| Chemicals |  ChemComp-MG:  ChemComp-CA:  ChemComp-TPP:  ChemComp-EPE:  ChemComp-COA:  ChemComp-ACO:  ChemComp-HOH:  ChemComp-SCA:  ChemComp-QSP: |
| Source |
|
 Keywords Keywords |  OXIDOREDUCTASE / OXIDOREDUCTASE /  2-oxoglutarate dehydrogenase; ODH; KGD; OXIDOREDUCTASE / 2-oxoglutarate dehydrogenase; ODH; KGD; OXIDOREDUCTASE /  2-oxoglutarate dehydrogenase; ODH 2-oxoglutarate dehydrogenase; ODH |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers