+Search query
-Structure paper
| Title | Structure and flexibility of the yeast NuA4 histone acetyltransferase complex. |
|---|---|
| Journal, issue, pages | Elife, Vol. 11, Year 2022 |
| Publish date | Oct 20, 2022 |
 Authors Authors | Stefan A Zukin / Matthew R Marunde / Irina K Popova / Katarzyna M Soczek / Eva Nogales / Avinash B Patel /  |
| PubMed Abstract | The NuA4 protein complex acetylates histones H4 and H2A to activate both transcription and DNA repair. We report the 3.1-Å resolution cryo-electron microscopy structure of the central hub of NuA4, ...The NuA4 protein complex acetylates histones H4 and H2A to activate both transcription and DNA repair. We report the 3.1-Å resolution cryo-electron microscopy structure of the central hub of NuA4, which flexibly tethers the histone acetyltransferase (HAT) and Trimer Independent of NuA4 involved in Transcription Interactions with Nucleosomes (TINTIN) modules. The hub contains the large Tra1 subunit and a core that includes Swc4, Arp4, Act1, Eaf1, and the C-terminal region of Epl1. Eaf1 stands out as the primary scaffolding factor that interacts with the Tra1, Swc4, and Epl1 subunits and contributes the conserved HSA helix to the Arp module. Using nucleosome-binding assays, we find that the HAT module, which is anchored to the core through Epl1, recognizes H3K4me3 nucleosomes with hyperacetylated H3 tails, while the TINTIN module, anchored to the core via Eaf1, recognizes nucleosomes that have hyperacetylated H2A and H4 tails. Together with the known interaction of Tra1 with site-specific transcription factors, our data suggest a model in which Tra1 recruits NuA4 to specific genomic sites then allowing the flexible HAT and TINTIN modules to select nearby nucleosomes for acetylation. |
 External links External links |  Elife / Elife /  PubMed:36263929 / PubMed:36263929 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.9 - 3.9 Å |
| Structure data |  EMDB-28563: NuA4 core 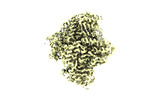 EMDB-28565: NuA4 FATKIN 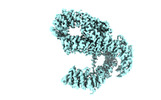 EMDB-28566: NuA4 HEAT  EMDB-28567: NuA4 HEAT bottom 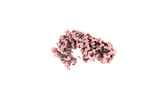 EMDB-28568: NuA4 HEAT top 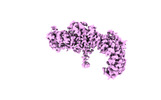 EMDB-28569: NuA4 HEAT middle EMDB-28575, PDB-8esc: |
| Chemicals |  ChemComp-ATP:  ChemComp-MG: |
| Source |
|
 Keywords Keywords |  TRANSCRIPTION / TRANSCRIPTION /  Histone Acetyltransferase / NuA4 / Histone Acetyltransferase / NuA4 /  Yeast Yeast |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers






