+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8esc | ||||||
|---|---|---|---|---|---|---|---|
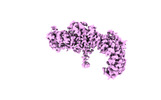
| Title | Structure of the Yeast NuA4 Histone Acetyltransferase Complex | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  TRANSCRIPTION / TRANSCRIPTION /  Histone Acetyltransferase / NuA4 / Histone Acetyltransferase / NuA4 /  Yeast Yeast | ||||||
| Function / homology |  Function and homology information Function and homology informationmitotic actomyosin contractile ring contraction / RHOA GTPase cycle / cellular bud neck contractile ring / piccolo histone acetyltransferase complex / RHO GTPases activate IQGAPs / RHO GTPases Activate WASPs and WAVEs / Regulation of actin dynamics for phagocytic cup formation / ascospore wall assembly / vacuole inheritance / actin cortical patch ...mitotic actomyosin contractile ring contraction / RHOA GTPase cycle / cellular bud neck contractile ring / piccolo histone acetyltransferase complex / RHO GTPases activate IQGAPs / RHO GTPases Activate WASPs and WAVEs / Regulation of actin dynamics for phagocytic cup formation / ascospore wall assembly / vacuole inheritance / actin cortical patch / Swr1 complex / Platelet degranulation / : / SLIK (SAGA-like) complex / Ino80 complex /  kinetochore assembly / kinetochore assembly /  SWI/SNF complex / SAGA complex / SWI/SNF complex / SAGA complex /  NuA4 histone acetyltransferase complex / establishment of cell polarity / actin filament bundle / positive regulation of macroautophagy / NuA4 histone acetyltransferase complex / establishment of cell polarity / actin filament bundle / positive regulation of macroautophagy /  protein secretion / chromosome organization / protein secretion / chromosome organization /  actin filament / actin filament /  Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / structural constituent of cytoskeleton / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / structural constituent of cytoskeleton /  endocytosis / transcription corepressor activity / endocytosis / transcription corepressor activity /  nucleosome / chromatin organization / nucleosome / chromatin organization /  histone binding / protein-containing complex assembly / histone binding / protein-containing complex assembly /  hydrolase activity / hydrolase activity /  chromatin remodeling / chromatin remodeling /  cell cycle / cell cycle /  DNA repair / DNA-templated transcription / DNA repair / DNA-templated transcription /  chromatin binding / chromatin binding /  chromatin / regulation of DNA-templated transcription / regulation of transcription by RNA polymerase II / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II / chromatin / regulation of DNA-templated transcription / regulation of transcription by RNA polymerase II / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II /  ATP binding / identical protein binding / ATP binding / identical protein binding /  nucleus nucleusSimilarity search - Function | ||||||
| Biological species |   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast) | ||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.1 Å cryo EM / Resolution: 3.1 Å | ||||||
 Authors Authors | Patel, A.B. / Zukin, S.A. / Nogales, E. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Elife / Year: 2022 Journal: Elife / Year: 2022Title: Structure and flexibility of the yeast NuA4 histone acetyltransferase complex. Authors: Stefan A Zukin / Matthew R Marunde / Irina K Popova / Katarzyna M Soczek / Eva Nogales / Avinash B Patel /  Abstract: The NuA4 protein complex acetylates histones H4 and H2A to activate both transcription and DNA repair. We report the 3.1-Å resolution cryo-electron microscopy structure of the central hub of NuA4, ...The NuA4 protein complex acetylates histones H4 and H2A to activate both transcription and DNA repair. We report the 3.1-Å resolution cryo-electron microscopy structure of the central hub of NuA4, which flexibly tethers the histone acetyltransferase (HAT) and Trimer Independent of NuA4 involved in Transcription Interactions with Nucleosomes (TINTIN) modules. The hub contains the large Tra1 subunit and a core that includes Swc4, Arp4, Act1, Eaf1, and the C-terminal region of Epl1. Eaf1 stands out as the primary scaffolding factor that interacts with the Tra1, Swc4, and Epl1 subunits and contributes the conserved HSA helix to the Arp module. Using nucleosome-binding assays, we find that the HAT module, which is anchored to the core through Epl1, recognizes H3K4me3 nucleosomes with hyperacetylated H3 tails, while the TINTIN module, anchored to the core via Eaf1, recognizes nucleosomes that have hyperacetylated H2A and H4 tails. Together with the known interaction of Tra1 with site-specific transcription factors, our data suggest a model in which Tra1 recruits NuA4 to specific genomic sites then allowing the flexible HAT and TINTIN modules to select nearby nucleosomes for acetylation. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8esc.cif.gz 8esc.cif.gz | 1002.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8esc.ent.gz pdb8esc.ent.gz | 780.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8esc.json.gz 8esc.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/es/8esc https://data.pdbj.org/pub/pdb/validation_reports/es/8esc ftp://data.pdbj.org/pub/pdb/validation_reports/es/8esc ftp://data.pdbj.org/pub/pdb/validation_reports/es/8esc | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  28575MC C: citing same article ( M: map data used to model this data |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 6 types, 6 molecules ZRAPES
| #1: Protein | Mass: 433677.281 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Saccharomyces cerevisiae (brewer's yeast) / References: UniProt: P38811 Saccharomyces cerevisiae (brewer's yeast) / References: UniProt: P38811 |
|---|---|
| #2: Protein | Mass: 54894.684 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Saccharomyces cerevisiae (brewer's yeast) / References: UniProt: P80428 Saccharomyces cerevisiae (brewer's yeast) / References: UniProt: P80428 |
| #3: Protein |  Mass: 41735.547 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast)References: UniProt: P60010,  Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement |
| #4: Protein | Mass: 96889.867 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Saccharomyces cerevisiae (brewer's yeast) / References: UniProt: P43572 Saccharomyces cerevisiae (brewer's yeast) / References: UniProt: P43572 |
| #5: Protein | Mass: 112667.008 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Saccharomyces cerevisiae (brewer's yeast) / References: UniProt: Q06337 Saccharomyces cerevisiae (brewer's yeast) / References: UniProt: Q06337 |
| #6: Protein | Mass: 55297.684 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Saccharomyces cerevisiae (brewer's yeast) / References: UniProt: P53201 Saccharomyces cerevisiae (brewer's yeast) / References: UniProt: P53201 |
-Non-polymers , 2 types, 3 molecules 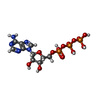


| #7: Chemical | ChemComp-ATP /  Adenosine triphosphate Adenosine triphosphate |
|---|---|
| #8: Chemical |
-Details
| Has ligand of interest | N |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: NuA4 / Type: COMPLEX / Entity ID: #1-#6 / Source: NATURAL |
|---|---|
| Molecular weight | Value: 1.3 MDa / Experimental value: NO |
| Source (natural) | Organism:   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast) |
| Buffer solution | pH: 7.9 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
| Specimen support | Grid material: GOLD / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R1.2/1.3 |
Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TECNAI ARCTICA |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 1500 nm / Nominal defocus min: 500 nm Bright-field microscopy / Nominal defocus max: 1500 nm / Nominal defocus min: 500 nm |
| Image recording | Electron dose: 40 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software | Name: SerialEM / Category: image acquisition | ||||||||||||||||||||||||
CTF correction | Type: NONE | ||||||||||||||||||||||||
3D reconstruction | Resolution: 3.1 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 635860 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refinement | Cross valid method: NONE Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2 | ||||||||||||||||||||||||
| Displacement parameters | Biso mean: 66.87 Å2 | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller





 PDBj
PDBj






