+Search query
-Structure paper
| Title | Filament structure of bacterial tubulin homologue TubZ. |
|---|---|
| Journal, issue, pages | Proc Natl Acad Sci U S A, Vol. 107, Issue 46, Page 19766-19771, Year 2010 |
| Publish date | Nov 16, 2010 |
 Authors Authors | Christopher H S Aylett / Qing Wang / Katharine A Michie / Linda A Amos / Jan Löwe /  |
| PubMed Abstract | Low copy number plasmids often depend on accurate partitioning systems for their continued survival. Generally, such systems consist of a centromere-like region of DNA, a DNA-binding adaptor, and a ...Low copy number plasmids often depend on accurate partitioning systems for their continued survival. Generally, such systems consist of a centromere-like region of DNA, a DNA-binding adaptor, and a polymerizing cytomotive filament. Together these components drive newly replicated plasmids to opposite ends of the dividing cell. The Bacillus thuringiensis plasmid pBToxis relies on a filament of the tubulin/FtsZ-like protein TubZ for its segregation. By combining crystallography and electron microscopy, we have determined the structure of this filament. We explain how GTP hydrolysis weakens the subunit-subunit contact and also shed light on the partitioning of the plasmid-adaptor complex. The double helical superstructure of TubZ filaments is unusual for tubulin-like proteins. Filaments of ParM, the actin-like partitioning protein, are also double helical. We suggest that convergent evolution shapes these different types of cytomotive filaments toward a general mechanism for plasmid separation. |
 External links External links |  Proc Natl Acad Sci U S A / Proc Natl Acad Sci U S A /  PubMed:20974911 / PubMed:20974911 /  PubMed Central PubMed Central |
| Methods | EM (helical sym.) / X-ray diffraction |
| Resolution | 3 - 35.0 Å |
| Structure data |  EMDB-1757:  EMDB-1758:  EMDB-1759:  EMDB-1760:  PDB-2xka:  PDB-2xkb: |
| Chemicals | 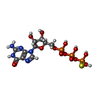 ChemComp-GSP:  ChemComp-MG: 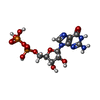 ChemComp-GDP: |
| Source |
|
 Keywords Keywords |  STRUCTURAL PROTEIN / STRUCTURAL PROTEIN /  MOTOR PROTEIN / MOTOR PROTEIN /  CYTOSKELETON / CYTOMOTIVE / DNA SEGREGATION / CYTOSKELETON / CYTOMOTIVE / DNA SEGREGATION /  MICROTUBULE / PBTOXIS / PBT156 / REPX / MICROTUBULE / PBTOXIS / PBT156 / REPX /  TUBR TUBR |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers




