+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7drt | ||||||
|---|---|---|---|---|---|---|---|
| Title | Human Wntless in complex with Wnt3a | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  MEMBRANE PROTEIN / MEMBRANE PROTEIN /  Wnt signaling / Wnt secretion / WLS / Wnt3a / Wnt signaling / Wnt secretion / WLS / Wnt3a /  cryo-EM / palmitoleoylation cryo-EM / palmitoleoylation | ||||||
| Function / homology |  Function and homology information Function and homology informationWnt signaling pathway involved in forebrain neuroblast division / positive regulation of dermatome development / axis elongation involved in somitogenesis / calcium ion transmembrane transport via low voltage-gated calcium channel / : / positive regulation of collateral sprouting in absence of injury / positive regulation of mesodermal cell fate specification / paraxial mesodermal cell fate commitment /  cell proliferation in midbrain / spinal cord association neuron differentiation ...Wnt signaling pathway involved in forebrain neuroblast division / positive regulation of dermatome development / axis elongation involved in somitogenesis / calcium ion transmembrane transport via low voltage-gated calcium channel / : / positive regulation of collateral sprouting in absence of injury / positive regulation of mesodermal cell fate specification / paraxial mesodermal cell fate commitment / cell proliferation in midbrain / spinal cord association neuron differentiation ...Wnt signaling pathway involved in forebrain neuroblast division / positive regulation of dermatome development / axis elongation involved in somitogenesis / calcium ion transmembrane transport via low voltage-gated calcium channel / : / positive regulation of collateral sprouting in absence of injury / positive regulation of mesodermal cell fate specification / paraxial mesodermal cell fate commitment /  cell proliferation in midbrain / spinal cord association neuron differentiation / Formation of the posterior neural plate / Wnt protein secretion / cell proliferation in midbrain / spinal cord association neuron differentiation / Formation of the posterior neural plate / Wnt protein secretion /  COP9 signalosome assembly / Wnt-Frizzled-LRP5/6 complex / positive regulation of cell-cell adhesion mediated by cadherin / positive regulation of Wnt protein secretion / Negative regulation of TCF-dependent signaling by WNT ligand antagonists / COP9 signalosome assembly / Wnt-Frizzled-LRP5/6 complex / positive regulation of cell-cell adhesion mediated by cadherin / positive regulation of Wnt protein secretion / Negative regulation of TCF-dependent signaling by WNT ligand antagonists /  synaptic vesicle recycling / Signaling by RNF43 mutants / WNT ligand biogenesis and trafficking / positive regulation of cardiac muscle cell differentiation / synaptic vesicle recycling / Signaling by RNF43 mutants / WNT ligand biogenesis and trafficking / positive regulation of cardiac muscle cell differentiation /  cell proliferation in forebrain / cell proliferation in forebrain /  secondary palate development / negative regulation of axon extension involved in axon guidance / cementum mineralization / somatic stem cell division / non-canonical Wnt signaling pathway / secondary palate development / negative regulation of axon extension involved in axon guidance / cementum mineralization / somatic stem cell division / non-canonical Wnt signaling pathway /  co-receptor binding / cardiac muscle cell fate commitment / presynapse assembly / hindbrain development / positive regulation of skeletal muscle tissue development / Wnt-protein binding / dorsal/ventral neural tube patterning / negative regulation of dopaminergic neuron differentiation / midbrain dopaminergic neuron differentiation / regulation of postsynapse to nucleus signaling pathway / post-anal tail morphogenesis / exocrine pancreas development / co-receptor binding / cardiac muscle cell fate commitment / presynapse assembly / hindbrain development / positive regulation of skeletal muscle tissue development / Wnt-protein binding / dorsal/ventral neural tube patterning / negative regulation of dopaminergic neuron differentiation / midbrain dopaminergic neuron differentiation / regulation of postsynapse to nucleus signaling pathway / post-anal tail morphogenesis / exocrine pancreas development /  mammary gland development / positive regulation of neural precursor cell proliferation / mammary gland development / positive regulation of neural precursor cell proliferation /  frizzled binding / positive regulation of hepatocyte proliferation / Class B/2 (Secretin family receptors) / myoblast differentiation / Disassembly of the destruction complex and recruitment of AXIN to the membrane / anterior/posterior axis specification / inner ear morphogenesis / negative regulation of fat cell differentiation / midbrain development / regulation of synapse organization / fat cell differentiation / heart looping / B cell proliferation / Formation of paraxial mesoderm / skeletal muscle cell differentiation / positive regulation of receptor internalization / frizzled binding / positive regulation of hepatocyte proliferation / Class B/2 (Secretin family receptors) / myoblast differentiation / Disassembly of the destruction complex and recruitment of AXIN to the membrane / anterior/posterior axis specification / inner ear morphogenesis / negative regulation of fat cell differentiation / midbrain development / regulation of synapse organization / fat cell differentiation / heart looping / B cell proliferation / Formation of paraxial mesoderm / skeletal muscle cell differentiation / positive regulation of receptor internalization /  hemopoiesis / regulation of presynapse assembly / mesoderm formation / positive regulation of Wnt signaling pathway / canonical Wnt signaling pathway / hemopoiesis / regulation of presynapse assembly / mesoderm formation / positive regulation of Wnt signaling pathway / canonical Wnt signaling pathway /  endomembrane system / endomembrane system /  cell fate commitment / regulation of microtubule cytoskeleton organization / cellular response to retinoic acid / positive regulation of B cell proliferation / Regulation of FZD by ubiquitination / extracellular matrix organization / TCF dependent signaling in response to WNT / cell fate commitment / regulation of microtubule cytoskeleton organization / cellular response to retinoic acid / positive regulation of B cell proliferation / Regulation of FZD by ubiquitination / extracellular matrix organization / TCF dependent signaling in response to WNT /  cytokine activity / cytokine activity /  axon guidance / positive regulation of cytokine production / hippocampus development / positive regulation of protein localization to plasma membrane / axon guidance / positive regulation of cytokine production / hippocampus development / positive regulation of protein localization to plasma membrane /  intracellular protein transport / modulation of chemical synaptic transmission / intracellular protein transport / modulation of chemical synaptic transmission /  trans-Golgi network / trans-Golgi network /  protein localization / neuron differentiation / protein localization / neuron differentiation /  platelet aggregation / platelet aggregation /  Wnt signaling pathway / osteoblast differentiation / negative regulation of neurogenesis / Golgi lumen / positive regulation of canonical Wnt signaling pathway / endocytic vesicle membrane / presynapse / negative regulation of neuron projection development / positive regulation of peptidyl-serine phosphorylation / Wnt signaling pathway / osteoblast differentiation / negative regulation of neurogenesis / Golgi lumen / positive regulation of canonical Wnt signaling pathway / endocytic vesicle membrane / presynapse / negative regulation of neuron projection development / positive regulation of peptidyl-serine phosphorylation /  heart development / cytoplasmic vesicle / early endosome membrane / positive regulation of canonical NF-kappaB signal transduction / in utero embryonic development / cell population proliferation / transcription by RNA polymerase II / heart development / cytoplasmic vesicle / early endosome membrane / positive regulation of canonical NF-kappaB signal transduction / in utero embryonic development / cell population proliferation / transcription by RNA polymerase II /  transcription coactivator activity / transcription coactivator activity /  receptor ligand activity / receptor ligand activity /  early endosome early endosomeSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.2 Å cryo EM / Resolution: 2.2 Å | ||||||
 Authors Authors | Zhong, Q. / Zhao, Y. / Ye, F. / Xiao, Z. / Huang, G. / Zhang, Y. / Lu, P. / Xu, W. / Zhou, Q. / Ma, D. | ||||||
| Funding support |  China, 1items China, 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: Cryo-EM structure of human Wntless in complex with Wnt3a. Authors: Qing Zhong / Yanyu Zhao / Fangfei Ye / Zaiyu Xiao / Gaoxingyu Huang / Meng Xu / Yuanyuan Zhang / Xiechao Zhan / Ke Sun / Zhizhi Wang / Shanshan Cheng / Shan Feng / Xiuxiu Zhao / Jizhong ...Authors: Qing Zhong / Yanyu Zhao / Fangfei Ye / Zaiyu Xiao / Gaoxingyu Huang / Meng Xu / Yuanyuan Zhang / Xiechao Zhan / Ke Sun / Zhizhi Wang / Shanshan Cheng / Shan Feng / Xiuxiu Zhao / Jizhong Zhang / Peilong Lu / Wenqing Xu / Qiang Zhou / Dan Ma /  Abstract: Wntless (WLS), an evolutionarily conserved multi-pass transmembrane protein, is essential for secretion of Wnt proteins. Wnt-triggered signaling pathways control many crucial life events, whereas ...Wntless (WLS), an evolutionarily conserved multi-pass transmembrane protein, is essential for secretion of Wnt proteins. Wnt-triggered signaling pathways control many crucial life events, whereas aberrant Wnt signaling is tightly associated with many human diseases including cancers. Here, we report the cryo-EM structure of human WLS in complex with Wnt3a, the most widely studied Wnt, at 2.2 Å resolution. The transmembrane domain of WLS bears a GPCR fold, with a conserved core cavity and a lateral opening. Wnt3a interacts with WLS at multiple interfaces, with the lipid moiety on Wnt3a traversing a hydrophobic tunnel of WLS transmembrane domain and inserting into membrane. A β-hairpin of Wnt3a containing the conserved palmitoleoylation site interacts with WLS extensively, which is crucial for WLS-mediated Wnt secretion. The flexibility of the Wnt3a loop/hairpin regions involved in the multiple binding sites indicates induced fit might happen when Wnts are bound to different binding partners. Our findings provide important insights into the molecular mechanism of Wnt palmitoleoylation, secretion and signaling. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7drt.cif.gz 7drt.cif.gz | 157.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7drt.ent.gz pdb7drt.ent.gz | 128.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7drt.json.gz 7drt.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/dr/7drt https://data.pdbj.org/pub/pdb/validation_reports/dr/7drt ftp://data.pdbj.org/pub/pdb/validation_reports/dr/7drt ftp://data.pdbj.org/pub/pdb/validation_reports/dr/7drt | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  30827MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 2 types, 2 molecules AB
| #1: Protein | Mass: 39421.832 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: WNT3A / Cell line (production host): HEK293 / Production host: Homo sapiens (human) / Gene: WNT3A / Cell line (production host): HEK293 / Production host:   Homo sapiens (human) / References: UniProt: P56704 Homo sapiens (human) / References: UniProt: P56704 |
|---|---|
| #2: Protein | Mass: 62317.973 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: WLS, C1orf139, GPR177, UNQ85/PRO18667 / Cell line (production host): HEK293 / Production host: Homo sapiens (human) / Gene: WLS, C1orf139, GPR177, UNQ85/PRO18667 / Cell line (production host): HEK293 / Production host:   Homo sapiens (human) / References: UniProt: Q5T9L3 Homo sapiens (human) / References: UniProt: Q5T9L3 |
-Sugars , 1 types, 1 molecules
| #3: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose / Mass: 424.401 Da / Num. of mol.: 1 / Source method: obtained synthetically / Mass: 424.401 Da / Num. of mol.: 1 / Source method: obtained synthetically |
|---|
-Non-polymers , 5 types, 15 molecules 

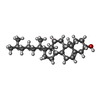






| #4: Chemical | ChemComp-PAM /  Palmitoleic acid Palmitoleic acid | ||||||
|---|---|---|---|---|---|---|---|
| #5: Chemical | | #6: Chemical | ChemComp-CLR / |  Cholesterol Cholesterol#7: Chemical | ChemComp-LPE / | #8: Water | ChemComp-HOH / |  Water Water |
-Details
| Has ligand of interest | N |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source (natural) |
| ||||||||||||||||||||||||
| Source (recombinant) |
| ||||||||||||||||||||||||
| Buffer solution | pH: 8 | ||||||||||||||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES | ||||||||||||||||||||||||
Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Alignment procedure: COMA FREE Bright-field microscopy / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) |
- Processing
Processing
| EM software | Name: RELION / Version: 3.0.6 / Category: 3D reconstruction |
|---|---|
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
3D reconstruction | Resolution: 2.2 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 8482332 / Symmetry type: POINT |
 Movie
Movie Controller
Controller













 PDBj
PDBj











