Entry Database : PDB / ID : 4hqjTitle Crystal structure of Na+,K+-ATPase in the Na+-bound state (Sodium/potassium-transporting ATPase subunit ...) x 2 Na+/K+ ATPase gamma subunit transcript variant a Keywords / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Sus scrofa (pig)Method / / / Resolution : 4.3 Å Authors Nyblom, M. / Reinhard, L. / Gourdon, P. / Nissen, P. Journal : Science / Year : 2013Title : Crystal structure of Na+, K(+)-ATPase in the Na(+)-bound state.Authors : Nyblom, M. / Poulsen, H. / Gourdon, P. / Reinhard, L. / Andersson, M. / Lindahl, E. / Fedosova, N. / Nissen, P. History Deposition Oct 25, 2012 Deposition site / Processing site Revision 1.0 Oct 2, 2013 Provider / Type Revision 1.1 Sep 10, 2014 Group
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Membrane protein / Na+/K+ transport / HYDROLASE-TRANSPORT PROTEIN complex
Membrane protein / Na+/K+ transport / HYDROLASE-TRANSPORT PROTEIN complex Function and homology information
Function and homology information Na+/K+-exchanging ATPase / positive regulation of sodium ion export across plasma membrane / positive regulation of potassium ion import across plasma membrane / Ion transport by P-type ATPases / P-type sodium:potassium-exchanging transporter activity / membrane repolarization /
Na+/K+-exchanging ATPase / positive regulation of sodium ion export across plasma membrane / positive regulation of potassium ion import across plasma membrane / Ion transport by P-type ATPases / P-type sodium:potassium-exchanging transporter activity / membrane repolarization /  sodium ion binding ...Basigin interactions / Ion homeostasis / positive regulation of P-type sodium:potassium-exchanging transporter activity /
sodium ion binding ...Basigin interactions / Ion homeostasis / positive regulation of P-type sodium:potassium-exchanging transporter activity /  Na+/K+-exchanging ATPase / positive regulation of sodium ion export across plasma membrane / positive regulation of potassium ion import across plasma membrane / Ion transport by P-type ATPases / P-type sodium:potassium-exchanging transporter activity / membrane repolarization /
Na+/K+-exchanging ATPase / positive regulation of sodium ion export across plasma membrane / positive regulation of potassium ion import across plasma membrane / Ion transport by P-type ATPases / P-type sodium:potassium-exchanging transporter activity / membrane repolarization /  sodium ion binding / sodium:potassium-exchanging ATPase complex / sodium ion export across plasma membrane / intracellular potassium ion homeostasis / establishment or maintenance of transmembrane electrochemical gradient / intracellular sodium ion homeostasis / potassium ion import across plasma membrane / ATPase activator activity /
sodium ion binding / sodium:potassium-exchanging ATPase complex / sodium ion export across plasma membrane / intracellular potassium ion homeostasis / establishment or maintenance of transmembrane electrochemical gradient / intracellular sodium ion homeostasis / potassium ion import across plasma membrane / ATPase activator activity /  potassium ion binding / sodium channel regulator activity / regulation of sodium ion transport / proton transmembrane transport /
potassium ion binding / sodium channel regulator activity / regulation of sodium ion transport / proton transmembrane transport /  sarcolemma / transmembrane transport /
sarcolemma / transmembrane transport /  melanosome / protein-macromolecule adaptor activity /
melanosome / protein-macromolecule adaptor activity /  ATPase binding / basolateral plasma membrane /
ATPase binding / basolateral plasma membrane /  cell adhesion / apical plasma membrane /
cell adhesion / apical plasma membrane /  axon /
axon /  innate immune response /
innate immune response /  ATP hydrolysis activity /
ATP hydrolysis activity /  ATP binding /
ATP binding /  membrane /
membrane /  plasma membrane
plasma membrane
 Sus scrofa (pig)
Sus scrofa (pig) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  SIRAS / Resolution: 4.3 Å
SIRAS / Resolution: 4.3 Å  Authors
Authors Citation
Citation Journal: Science / Year: 2013
Journal: Science / Year: 2013 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 4hqj.cif.gz
4hqj.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb4hqj.ent.gz
pdb4hqj.ent.gz PDB format
PDB format 4hqj.json.gz
4hqj.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/hq/4hqj
https://data.pdbj.org/pub/pdb/validation_reports/hq/4hqj ftp://data.pdbj.org/pub/pdb/validation_reports/hq/4hqj
ftp://data.pdbj.org/pub/pdb/validation_reports/hq/4hqj Links
Links Assembly
Assembly


 Components
Components
 Sus scrofa (pig) / References: UniProt: P05024, EC: 3.6.3.9
Sus scrofa (pig) / References: UniProt: P05024, EC: 3.6.3.9
 Sus scrofa (pig) / References: UniProt: P05027
Sus scrofa (pig) / References: UniProt: P05027
 Sus scrofa (pig) / References: UniProt: Q58K79
Sus scrofa (pig) / References: UniProt: Q58K79
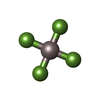


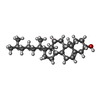
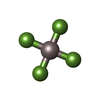



 Adenosine diphosphate
Adenosine diphosphate Cholesterol
Cholesterol X-RAY DIFFRACTION / Number of used crystals: 2
X-RAY DIFFRACTION / Number of used crystals: 2  Sample preparation
Sample preparation
 Processing
Processing :
:  SIRAS / Resolution: 4.3→11.999 Å / SU ML: 0.62 / σ(F): 2.01 / Phase error: 31.55 / Stereochemistry target values: MLHL
SIRAS / Resolution: 4.3→11.999 Å / SU ML: 0.62 / σ(F): 2.01 / Phase error: 31.55 / Stereochemistry target values: MLHL Movie
Movie Controller
Controller













 PDBj
PDBj



















