[English] 日本語
 Yorodumi
Yorodumi- PDB-1gpm: ESCHERICHIA COLI GMP SYNTHETASE COMPLEXED WITH AMP AND PYROPHOSPHATE -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1gpm | ||||||
|---|---|---|---|---|---|---|---|
| Title | ESCHERICHIA COLI GMP SYNTHETASE COMPLEXED WITH AMP AND PYROPHOSPHATE | ||||||
 Components Components | GMP SYNTHETASE GMP synthase GMP synthase | ||||||
 Keywords Keywords | TRANSFERASE (GLUTAMINE AMIDOTRANSFERASE) / CLASS I GLUTAMINE AMIDOTRANSFERASE / N-TYPE ATP PYROPHOSPHATASE | ||||||
| Function / homology |  Function and homology information Function and homology information GMP synthase activity / GMP synthase (glutamine-hydrolyzing) activity / GMP synthase activity / GMP synthase (glutamine-hydrolyzing) activity /  GMP synthase (glutamine-hydrolysing) / GMP synthase (glutamine-hydrolysing) /  pyrophosphatase activity / GMP biosynthetic process / glutamine metabolic process / pyrophosphatase activity / GMP biosynthetic process / glutamine metabolic process /  ATP binding / identical protein binding / ATP binding / identical protein binding /  cytosol cytosolSimilarity search - Function | ||||||
| Biological species |   Escherichia coli (E. coli) Escherichia coli (E. coli) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / Resolution: 2.2 Å SYNCHROTRON / Resolution: 2.2 Å | ||||||
 Authors Authors | Tesmer, J.J.G. | ||||||
 Citation Citation |  Journal: Nat.Struct.Biol. / Year: 1996 Journal: Nat.Struct.Biol. / Year: 1996Title: The crystal structure of GMP synthetase reveals a novel catalytic triad and is a structural paradigm for two enzyme families. Authors: Tesmer, J.J. / Klem, T.J. / Deras, M.L. / Davisson, V.J. / Smith, J.L. #1:  Journal: Proteins / Year: 1994 Journal: Proteins / Year: 1994Title: Preliminary X-Ray Analysis of Escherichia Coli Gmp Synthetase: Determination of Anomalous Scattering Factors for a Cysteinyl Mercury Derivative Authors: Tesmer, J.J.G. / Stemmler, T.L. / Penner-Hahn, J.E. / Davisson, V.J. / Smith, J.L. | ||||||
| History |
| ||||||
| Remark 650 | HELIX HELIX DETERMINATION METHOD: RESIDUES FORMING SECONDARY STRUCTURE HAVE BACKBONE H-BONDS < 3.4 ...HELIX HELIX DETERMINATION METHOD: RESIDUES FORMING SECONDARY STRUCTURE HAVE BACKBONE H-BONDS < 3.4 ANGSTROMS HELIX_ID: 5A,LAST 2 RESIDUES FORM PI H-BONDS. HELIX_ID: 8A,LAST 2 RESIDUES FORM PI H-BONDS. HELIX_ID: 5B,LAST 2 RESIDUES FORM PI H-BONDS. HELIX_ID: 8B,LAST 2 RESIDUES FORM PI H-BONDS. HELIX_ID: 5C,LAST 2 RESIDUES FORM PI H-BONDS. HELIX_ID: 8C,LAST 2 RESIDUES FORM PI H-BONDS. HELIX_ID: 5D,LAST 2 RESIDUES FORM PI H-BONDS. HELIX_ID: 8D,LAST 2 RESIDUES FORM PI H-BONDS. | ||||||
| Remark 700 | SHEET S1A, S1B, S1C, S1D: DETERMINATION METHOD: RESIDUES FORMING SECONDARY STRUCTURE HAVE BACKBONE ...SHEET S1A, S1B, S1C, S1D: DETERMINATION METHOD: RESIDUES FORMING SECONDARY STRUCTURE HAVE BACKBONE H-BONDS < 3.4 ANGSTROMS; S1A AND S2A, S1B AND S2B, S1C AND S2C, S1D AND S2D ARE CONTINUOUS, BUT THE OVERALL FOLD OF THE DOMAIN SUGGESTS THAT THEY ARE BETTER DESCRIBED AS DISTINCT SHEETS. THE FIRST STRAND IN S2A, S2B, S2C, S2D WOULD BE THE NEXT STRAND IN S1A S1B, S1C, S1D, RESPECTIVELY. S3A, S3B, S3C, S3D: BETA RIBBON. THE DIMERIZATION DOMAIN FOR CHAINS A AND C CONTAINS A SHEET WITH TWO BIFURCATED STRANDS, AS DOES THE DIMERIZATION DOMAIN FOR CHAINS B AND D. THESE SHEETS ARE PRESENTED AS SHEETS SAC AND TAC FOR CHAINS A AND C AND SHEETS SBD AND TBD FOR CHAINS B AND D. SHEETS SAC AND TAC DIFFER IN CHAINS 3 AND 4 AS DO SHEETS SBD AND TBD. SBD: DIMERIZATION DOMAIN. ATOM_NAME: () () CYS A 86 () CYS 86 HAS A STRAINED BACKBONE CONFORMATION (PHI=55, PSI= -110) TYPICAL OF "NUCLEOPHILE ELBOWS" FOUND IN THE A/B HYDROLASES. THE RESIDUE IS BOTH A MEMBER OF SHEET S1A AND HELIX 4A. ATOM_NAME: () () CYS B 86 () CYS 86 HAS A STRAINED BACKBONE CONFORMATION (PHI=55, PSI= -110) TYPICAL OF "NUCLEOPHILE ELBOWS" FOUND IN THE A/B HYDROLASES. THE RESIDUE IS BOTH A MEMBER OF SHEET S1B AND HELIX 4B. ATOM_NAME: () () CYS C 86 () CYS 86 HAS A STRAINED BACKBONE CONFORMATION (PHI=55, PSI= -110) TYPICAL OF "NUCLEOPHILE ELBOWS" FOUND IN THE A/B HYDROLASES. THE RESIDUE IS BOTH A MEMBER OF SHEET S1C AND HELIX 4C. ATOM_NAME: () () CYS D 86 () CYS 86 HAS A STRAINED BACKBONE CONFORMATION (PHI=55, PSI= -110) TYPICAL OF "NUCLEOPHILE ELBOWS" FOUND IN THE A/B HYDROLASES. THE RESIDUE IS BOTH A MEMBER OF SHEET S1D AND HELIX 4D. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1gpm.cif.gz 1gpm.cif.gz | 418 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1gpm.ent.gz pdb1gpm.ent.gz | 340.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1gpm.json.gz 1gpm.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gp/1gpm https://data.pdbj.org/pub/pdb/validation_reports/gp/1gpm ftp://data.pdbj.org/pub/pdb/validation_reports/gp/1gpm ftp://data.pdbj.org/pub/pdb/validation_reports/gp/1gpm | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Atom site foot note | 1: CIS PROLINE - PRO A 518 / 2: CIS PROLINE - PRO A 519 / 3: CIS PROLINE - PRO B 518 / 4: CIS PROLINE - PRO B 519 / 5: CIS PROLINE - PRO C 518 / 6: CIS PROLINE - PRO C 519 / 7: CIS PROLINE - PRO D 518 / 8: CIS PROLINE - PRO D 519 |
- Components
Components
-Protein , 1 types, 4 molecules ABCD
| #1: Protein |  GMP synthase / XMP AMINASE GMP synthase / XMP AMINASEMass: 58750.043 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Escherichia coli (E. coli) / Strain: K12 / Description: TAC PROMOTER / Gene: GUAA / Plasmid: PGUAA-TAC / Gene (production host): GUAA / Production host: Escherichia coli (E. coli) / Strain: K12 / Description: TAC PROMOTER / Gene: GUAA / Plasmid: PGUAA-TAC / Gene (production host): GUAA / Production host:   Escherichia coli (E. coli) Escherichia coli (E. coli)References: UniProt: P04079,  GMP synthase (glutamine-hydrolysing) GMP synthase (glutamine-hydrolysing) |
|---|
-Non-polymers , 6 types, 809 molecules 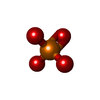


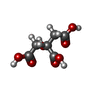







| #2: Chemical | ChemComp-PO4 /  Phosphate Phosphate#3: Chemical | ChemComp-POP /  Pyrophosphate Pyrophosphate#4: Chemical | ChemComp-AMP /  Adenosine monophosphate Adenosine monophosphate#5: Chemical | ChemComp-CIT /  Citric acid Citric acid#6: Chemical | #7: Water | ChemComp-HOH / |  Water Water |
|---|
-Details
| Sequence details | N-TERMINAL SEQUENCING |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Crystal grow | pH: 5 / Details: pH 5.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 20 ℃ / Method: vapor diffusion, sitting drop | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction |
| |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source |
| |||||||||||||||||||||
| Detector |
| |||||||||||||||||||||
| Radiation | Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||||||||
| Radiation wavelength |
| |||||||||||||||||||||
| Reflection | Resolution: 2.2→50 Å / Num. obs: 105224 / % possible obs: 84.5 % / Observed criterion σ(I): 0 / Redundancy: 4.2 % / Rmerge(I) obs: 0.053 | |||||||||||||||||||||
| Reflection | *PLUS % possible obs: 841.4 % / Rmerge(I) obs: 0.059 | |||||||||||||||||||||
| Reflection shell | *PLUS % possible obs: 48.4 % / Rmerge(I) obs: 0.149 / Mean I/σ(I) obs: 8.7 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.2→6 Å /
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 35.3 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.2→6 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Classification: refinement X-PLOR / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller








 PDBj
PDBj

















