[English] 日本語
 Yorodumi
Yorodumi- EMDB-18664: Structure of the native microtubule lattice nucleated from the ye... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the native microtubule lattice nucleated from the yeast spindle pole body | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords |  Microtubule nucleation / MTOC / y-tubulin / SPB / Microtubule nucleation / MTOC / y-tubulin / SPB /  CELL CYCLE CELL CYCLE | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationnuclear migration by microtubule mediated pushing forces /  Cilium Assembly / Cilium Assembly /  nuclear division / Sealing of the nuclear envelope (NE) by ESCRT-III / nuclear migration along microtubule / homologous chromosome segregation / Platelet degranulation / nuclear division / Sealing of the nuclear envelope (NE) by ESCRT-III / nuclear migration along microtubule / homologous chromosome segregation / Platelet degranulation /  tubulin complex / mitotic sister chromatid segregation / microtubule-based process ...nuclear migration by microtubule mediated pushing forces / tubulin complex / mitotic sister chromatid segregation / microtubule-based process ...nuclear migration by microtubule mediated pushing forces /  Cilium Assembly / Cilium Assembly /  nuclear division / Sealing of the nuclear envelope (NE) by ESCRT-III / nuclear migration along microtubule / homologous chromosome segregation / Platelet degranulation / nuclear division / Sealing of the nuclear envelope (NE) by ESCRT-III / nuclear migration along microtubule / homologous chromosome segregation / Platelet degranulation /  tubulin complex / mitotic sister chromatid segregation / microtubule-based process / nuclear periphery / tubulin complex / mitotic sister chromatid segregation / microtubule-based process / nuclear periphery /  Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / structural constituent of cytoskeleton / microtubule cytoskeleton organization / spindle / mitotic cell cycle / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / structural constituent of cytoskeleton / microtubule cytoskeleton organization / spindle / mitotic cell cycle /  microtubule / microtubule /  hydrolase activity / hydrolase activity /  GTPase activity / GTP binding / GTPase activity / GTP binding /  metal ion binding / metal ion binding /  nucleus / nucleus /  cytoplasm cytoplasmSimilarity search - Function | ||||||||||||
| Biological species |   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast) | ||||||||||||
| Method | subtomogram averaging /  cryo EM / Resolution: 6.6 Å cryo EM / Resolution: 6.6 Å | ||||||||||||
 Authors Authors | Dendooven T / Yatskevich S / Burt A / Bellini D / Kilmartin J / Barford D | ||||||||||||
| Funding support |  United Kingdom, United Kingdom,  Germany, 3 items Germany, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2024 Journal: Nat Struct Mol Biol / Year: 2024Title: Structure of the native γ-tubulin ring complex capping spindle microtubules. Authors: Tom Dendooven / Stanislau Yatskevich / Alister Burt / Zhuo A Chen / Dom Bellini / Juri Rappsilber / John V Kilmartin / David Barford /    Abstract: Microtubule (MT) filaments, composed of α/β-tubulin dimers, are fundamental to cellular architecture, function and organismal development. They are nucleated from MT organizing centers by the ...Microtubule (MT) filaments, composed of α/β-tubulin dimers, are fundamental to cellular architecture, function and organismal development. They are nucleated from MT organizing centers by the evolutionarily conserved γ-tubulin ring complex (γTuRC). However, the molecular mechanism of nucleation remains elusive. Here we used cryo-electron tomography to determine the structure of the native γTuRC capping the minus end of a MT in the context of enriched budding yeast spindles. In our structure, γTuRC presents a ring of γ-tubulin subunits to seed nucleation of exclusively 13-protofilament MTs, adopting an active closed conformation to function as a perfect geometric template for MT nucleation. Our cryo-electron tomography reconstruction revealed that a coiled-coil protein staples the first row of α/β-tubulin of the MT to alternating positions along the γ-tubulin ring of γTuRC. This positioning of α/β-tubulin onto γTuRC suggests a role for the coiled-coil protein in augmenting γTuRC-mediated MT nucleation. Based on our results, we describe a molecular model for budding yeast γTuRC activation and MT nucleation. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_18664.map.gz emd_18664.map.gz | 34.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-18664-v30.xml emd-18664-v30.xml emd-18664.xml emd-18664.xml | 15.2 KB 15.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_18664.png emd_18664.png | 117 KB | ||
| Filedesc metadata |  emd-18664.cif.gz emd-18664.cif.gz | 5.9 KB | ||
| Others |  emd_18664_half_map_1.map.gz emd_18664_half_map_1.map.gz emd_18664_half_map_2.map.gz emd_18664_half_map_2.map.gz | 18.4 MB 18.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-18664 http://ftp.pdbj.org/pub/emdb/structures/EMD-18664 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18664 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18664 | HTTPS FTP |
-Related structure data
| Related structure data |  8qv0MC  8qv2C  8qv3C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_18664.map.gz / Format: CCP4 / Size: 36.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_18664.map.gz / Format: CCP4 / Size: 36.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 3.3 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_18664_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
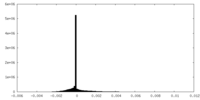
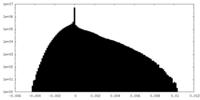
| Density Histograms |
-Half map: #1
| File | emd_18664_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
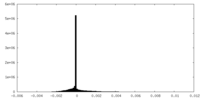
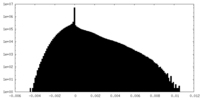
| Density Histograms |
- Sample components
Sample components
-Entire : y-Tubulin Ring Complex capping the microtubule minus end
| Entire | Name: y-Tubulin Ring Complex capping the microtubule minus end |
|---|---|
| Components |
|
-Supramolecule #1: y-Tubulin Ring Complex capping the microtubule minus end
| Supramolecule | Name: y-Tubulin Ring Complex capping the microtubule minus end type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast) |
-Macromolecule #1: Tubulin alpha-1 chain
| Macromolecule | Name: Tubulin alpha-1 chain / type: protein_or_peptide / ID: 1 / Number of copies: 13 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast) |
| Molecular weight | Theoretical: 49.853867 KDa |
| Sequence | String: MREVISINVG QAGCQIGNAC WELYSLEHGI KPDGHLEDGL SKPKGGEEGF STFFHETGYG KFVPRAIYVD LEPNVIDEVR NGPYKDLFH PEQLISGKED AANNYARGHY TVGREILGDV LDRIRKLADQ CDGLQGFLFT HSLGGGTGSG LGSLLLEELS A EYGKKSKL ...String: MREVISINVG QAGCQIGNAC WELYSLEHGI KPDGHLEDGL SKPKGGEEGF STFFHETGYG KFVPRAIYVD LEPNVIDEVR NGPYKDLFH PEQLISGKED AANNYARGHY TVGREILGDV LDRIRKLADQ CDGLQGFLFT HSLGGGTGSG LGSLLLEELS A EYGKKSKL EFAVYPAPQV STSVVEPYNT VLTTHTTLEH ADCTFMVDNE AIYDMCKRNL DIPRPSFANL NNLIAQVVSS VT ASLRFDG SLNVDLNEFQ TNLVPYPRIH FPLVSYSPVL SKSKAFHESN SVSEITNACF EPGNQMVKCD PRDGKYMATC LLY RGDVVT RDVQRAVEQV KNKKTVQLVD WCPTGFKIGI CYEPPTATPN SQLATVDRAV CMLSNTTSIA EAWKRIDRKF DLMY AKRAF VHWYVGEGME EGEFTEARED LAALERDYIE VGADSYAEEE EF UniProtKB: Tubulin alpha-1 chain |
-Macromolecule #2: Tubulin beta chain
| Macromolecule | Name: Tubulin beta chain / type: protein_or_peptide / ID: 2 / Number of copies: 13 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast) |
| Molecular weight | Theoretical: 50.967457 KDa |
| Sequence | String: MREIIHISTG QCGNQIGAAF WETICGEHGL DFNGTYHGHD DIQKERLNVY FNEASSGKWV PRSINVDLEP GTIDAVRNSA IGNLFRPDN YIFGQSSAGN VWAKGHYTEG AELVDSVMDV IRREAEGCDS LQGFQITHSL GGGTGSGMGT LLISKIREEF P DRMMATFS ...String: MREIIHISTG QCGNQIGAAF WETICGEHGL DFNGTYHGHD DIQKERLNVY FNEASSGKWV PRSINVDLEP GTIDAVRNSA IGNLFRPDN YIFGQSSAGN VWAKGHYTEG AELVDSVMDV IRREAEGCDS LQGFQITHSL GGGTGSGMGT LLISKIREEF P DRMMATFS VLPSPKTSDT VVEPYNATLS VHQLVEHSDE TFCIDNEALY DICQRTLKLN QPSYGDLNNL VSSVMSGVTT SL RYPGQLN SDLRKLAVNL VPFPRLHFFM VGYAPLTAIG SQSFRSLTVP ELTQQMFDAK NMMAAADPRN GRYLTVAAFF RGK VSVKEV EDEMHKVQSK NSDYFVEWIP NNVQTAVCSV APQGLDMAAT FIANSTSIQE LFKRVGDQFS AMFKRKAFLH WYTS EGMDE LEFSEAESNM NDLVSEYQQY QEATVEDDEE VDENGDFGAP QNQDEPITEN FE UniProtKB: Tubulin beta chain |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 6.53 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 4.5 µm / Nominal defocus min: 2.0 µm Bright-field microscopy / Nominal defocus max: 4.5 µm / Nominal defocus min: 2.0 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 3.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Extraction | Number tomograms: 364 / Number images used: 31720 |
|---|---|
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 6.6 Å / Resolution method: FSC 0.143 CUT-OFF / Number subtomograms used: 130704 |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)