+Search query
-Structure paper
| Title | Structure of ATP synthase from ESKAPE pathogen . |
|---|---|
| Journal, issue, pages | Sci Adv, Vol. 8, Issue 7, Page eabl5966, Year 2022 |
| Publish date | Feb 18, 2022 |
 Authors Authors | Julius K Demmer / Ben P Phillips / O Lisa Uhrig / Alain Filloux / Luke P Allsopp / Maike Bublitz / Thomas Meier /  |
| PubMed Abstract | The global spread of multidrug-resistant infections urgently calls for the identification of novel drug targets. We solved the electron cryo-microscopy structure of the FF-adenosine 5'-triphosphate ...The global spread of multidrug-resistant infections urgently calls for the identification of novel drug targets. We solved the electron cryo-microscopy structure of the FF-adenosine 5'-triphosphate (ATP) synthase from in three distinct conformational states. The nucleotide-converting F subcomplex reveals a specific self-inhibition mechanism, which supports a unidirectional ratchet mechanism to avoid wasteful ATP consumption. In the membrane-embedded F complex, the structure shows unique structural adaptations along both the entry and exit pathways of the proton-conducting a-subunit. These features, absent in mitochondrial ATP synthases, represent attractive targets for the development of next-generation therapeutics that can act directly at the culmination of bioenergetics in this clinically relevant pathogen. |
 External links External links |  Sci Adv / Sci Adv /  PubMed:35171679 / PubMed:35171679 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 3.1 - 4.6 Å |
| Structure data | EMDB-13174, PDB-7p2y: EMDB-13181, PDB-7p3n: EMDB-13186, PDB-7p3w: |
| Chemicals | 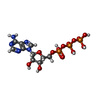 ChemComp-ATP:  ChemComp-MG:  ChemComp-ADP: 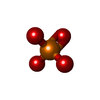 ChemComp-PO4:  ChemComp-HOH: |
| Source |
|
 Keywords Keywords |  MEMBRANE PROTEIN / MEMBRANE PROTEIN /  ATP synthase / ATP synthase /  ESKAPE / Rotary ATP synthase / F1Fo / peptidisc / ESKAPE / Rotary ATP synthase / F1Fo / peptidisc /  bioenergetics / IMP / bioenergetics / IMP /  multi-drug resistance / multi-drug resistance /  pathogenic pathogenic |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers










