+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8jni | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
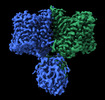
| Title | Structure of AE2 in complex with PIP2 | |||||||||
 Components Components | Anion exchange protein 2 | |||||||||
 Keywords Keywords |  MEMBRANE PROTEIN / AE2 / SLC4A2 / MEMBRANE PROTEIN / AE2 / SLC4A2 /  PIP2 PIP2 | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of CD8-positive, alpha-beta T cell differentiation / negative regulation of CD8-positive, alpha-beta T cell proliferation / positive regulation of enamel mineralization / Bicarbonate transporters /  amelogenesis / monoatomic anion transmembrane transporter activity / chloride:bicarbonate antiporter activity / solute:inorganic anion antiporter activity / bicarbonate transport / digestive tract development ...negative regulation of CD8-positive, alpha-beta T cell differentiation / negative regulation of CD8-positive, alpha-beta T cell proliferation / positive regulation of enamel mineralization / Bicarbonate transporters / amelogenesis / monoatomic anion transmembrane transporter activity / chloride:bicarbonate antiporter activity / solute:inorganic anion antiporter activity / bicarbonate transport / digestive tract development ...negative regulation of CD8-positive, alpha-beta T cell differentiation / negative regulation of CD8-positive, alpha-beta T cell proliferation / positive regulation of enamel mineralization / Bicarbonate transporters /  amelogenesis / monoatomic anion transmembrane transporter activity / chloride:bicarbonate antiporter activity / solute:inorganic anion antiporter activity / bicarbonate transport / digestive tract development / monoatomic anion transport / amelogenesis / monoatomic anion transmembrane transporter activity / chloride:bicarbonate antiporter activity / solute:inorganic anion antiporter activity / bicarbonate transport / digestive tract development / monoatomic anion transport /  regulation of bone resorption / transmembrane transporter activity / osteoclast differentiation / regulation of actin cytoskeleton organization / regulation of bone resorption / transmembrane transporter activity / osteoclast differentiation / regulation of actin cytoskeleton organization /  regulation of intracellular pH / transmembrane transport / regulation of intracellular pH / transmembrane transport /  spermatogenesis / basolateral plasma membrane / apical plasma membrane / spermatogenesis / basolateral plasma membrane / apical plasma membrane /  focal adhesion / focal adhesion /  enzyme binding / enzyme binding /  membrane / membrane /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.2 Å cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Yin, Y.X. / Ding, D. | |||||||||
| Funding support |  China, 2items China, 2items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structural and functional insights into the lipid regulation of human anion exchanger 2. Authors: Weiqi Zhang / Dian Ding / Yishuo Lu / Hongyi Chen / Peijun Jiang / Peng Zuo / Guangxi Wang / Juan Luo / Yue Yin / Jianyuan Luo / Yuxin Yin /  Abstract: Anion exchanger 2 (AE2) is an electroneutral Na-independent Cl/HCO exchanger belongs to the SLC4 transporter family. The widely expressed AE2 participates in a variety of physiological processes, ...Anion exchanger 2 (AE2) is an electroneutral Na-independent Cl/HCO exchanger belongs to the SLC4 transporter family. The widely expressed AE2 participates in a variety of physiological processes, including transepithelial acid-base secretion and osteoclastogenesis. Both the transmembrane domains (TMDs) and the N-terminal cytoplasmic domain (NTD) are involved in regulation of AE2 activity. However, the regulatory mechanism remains unclear. Here, we report a 3.2 Å cryo-EM structure of the AE2 TMDs in complex with PIP and a 3.3 Å full-length mutant AE2 structure in the resting state without PIP. We demonstrate that PIP at the TMD dimer interface is involved in the substrate exchange process. Mutation in the PIP binding site leads to the displacement of TM7 and further stabilizes the interaction between the TMD and the NTD. Reduced substrate transport activity and conformation similar to AE2 in acidic pH indicating the central contribution of PIP to the function of AE2. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8jni.cif.gz 8jni.cif.gz | 210.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8jni.ent.gz pdb8jni.ent.gz | 150 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8jni.json.gz 8jni.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jn/8jni https://data.pdbj.org/pub/pdb/validation_reports/jn/8jni ftp://data.pdbj.org/pub/pdb/validation_reports/jn/8jni ftp://data.pdbj.org/pub/pdb/validation_reports/jn/8jni | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  36448MC  8jnjC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein |  / AE 2 / Anion exchanger 2 / Non-erythroid band 3-like protein / BND3L / Solute carrier family 4 member 2 / AE 2 / Anion exchanger 2 / Non-erythroid band 3-like protein / BND3L / Solute carrier family 4 member 2Mass: 137176.906 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: SLC4A2, AE2, EPB3L1, HKB3, MPB3L / Production host: Homo sapiens (human) / Gene: SLC4A2, AE2, EPB3L1, HKB3, MPB3L / Production host:   Homo sapiens (human) / References: UniProt: P04920 Homo sapiens (human) / References: UniProt: P04920#2: Chemical |  Chloride Chloride#3: Chemical |  Phosphatidylinositol 4,5-bisphosphate Phosphatidylinositol 4,5-bisphosphateHas ligand of interest | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Anion exchange protein 2, isoform A Ion exchange / Type: COMPLEX / Entity ID: #1 / Source: RECOMBINANT Ion exchange / Type: COMPLEX / Entity ID: #1 / Source: RECOMBINANT |
|---|---|
| Molecular weight | Experimental value: NO |
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2000 nm / Nominal defocus min: 1800 nm Bright-field microscopy / Nominal defocus max: 2000 nm / Nominal defocus min: 1800 nm |
| Image recording | Electron dose: 52 e/Å2 / Detector mode: SUPER-RESOLUTION / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
- Processing
Processing
| EM software | Name: cryoSPARC / Version: 3.1.0 / Category: 3D reconstruction |
|---|---|
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
| Symmetry | Point symmetry : C2 (2 fold cyclic : C2 (2 fold cyclic ) ) |
3D reconstruction | Resolution: 3.2 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 107451 / Symmetry type: POINT |
 Movie
Movie Controller
Controller



 PDBj
PDBj



