[English] 日本語
 Yorodumi
Yorodumi- PDB-8bx8: In situ outer dynein arm from Chlamydomonas reinhardtii in the po... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8bx8 | ||||||
|---|---|---|---|---|---|---|---|
| Title | In situ outer dynein arm from Chlamydomonas reinhardtii in the post-power stroke state | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  MOTOR PROTEIN / MOTOR PROTEIN /  axoneme / outer dynein arm / post power stroke / axoneme / outer dynein arm / post power stroke /  dynein dynein | ||||||
| Function / homology |  Function and homology information Function and homology information dynein complex / minus-end-directed microtubule motor activity / dynein light intermediate chain binding / dynein complex / minus-end-directed microtubule motor activity / dynein light intermediate chain binding /  motile cilium / dynein intermediate chain binding / microtubule-based movement / microtubule-based process / motile cilium / dynein intermediate chain binding / microtubule-based movement / microtubule-based process /  microtubule / microtubule /  calcium ion binding / calcium ion binding /  ATP hydrolysis activity ... ATP hydrolysis activity ... dynein complex / minus-end-directed microtubule motor activity / dynein light intermediate chain binding / dynein complex / minus-end-directed microtubule motor activity / dynein light intermediate chain binding /  motile cilium / dynein intermediate chain binding / microtubule-based movement / microtubule-based process / motile cilium / dynein intermediate chain binding / microtubule-based movement / microtubule-based process /  microtubule / microtubule /  calcium ion binding / calcium ion binding /  ATP hydrolysis activity / ATP hydrolysis activity /  ATP binding / ATP binding /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Tetrahymena thermophila (eukaryote) Tetrahymena thermophila (eukaryote) | ||||||
| Method |  ELECTRON MICROSCOPY / subtomogram averaging / ELECTRON MICROSCOPY / subtomogram averaging /  cryo EM / Resolution: 30.3 Å cryo EM / Resolution: 30.3 Å | ||||||
 Authors Authors | Zimmermann, N.E.L. / Noga, A. / Obbineni, J.M. / Ishikawa, T. | ||||||
| Funding support |  Switzerland, 1items Switzerland, 1items
| ||||||
 Citation Citation |  Journal: EMBO J / Year: 2023 Journal: EMBO J / Year: 2023Title: ATP-induced conformational change of axonemal outer dynein arms revealed by cryo-electron tomography. Authors: Noemi Zimmermann / Akira Noga / Jagan Mohan Obbineni / Takashi Ishikawa /   Abstract: Axonemal outer dynein arm (ODA) motors generate force for ciliary beating. We analyzed three states of the ODA during the power stroke cycle using in situ cryo-electron tomography, subtomogram ...Axonemal outer dynein arm (ODA) motors generate force for ciliary beating. We analyzed three states of the ODA during the power stroke cycle using in situ cryo-electron tomography, subtomogram averaging, and classification. These states of force generation depict the prepower stroke, postpower stroke, and intermediate state conformations. Comparison of these conformations to published in vitro atomic structures of cytoplasmic dynein, ODA, and the Shulin-ODA complex revealed differences in the orientation and position of the dynein head. Our analysis shows that in the absence of ATP, all dynein linkers interact with the AAA3/AAA4 domains, indicating that interactions with the adjacent microtubule doublet B-tubule direct dynein orientation. For the prepower stroke conformation, there were changes in the tail that is anchored on the A-tubule. We built models starting with available high-resolution structures to generate a best-fitting model structure for the in situ pre- and postpower stroke ODA conformations, thereby showing that ODA in a complex with Shulin adopts a similar conformation as the active prepower stroke ODA in the axoneme. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8bx8.cif.gz 8bx8.cif.gz | 2.9 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8bx8.ent.gz pdb8bx8.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  8bx8.json.gz 8bx8.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/bx/8bx8 https://data.pdbj.org/pub/pdb/validation_reports/bx/8bx8 ftp://data.pdbj.org/pub/pdb/validation_reports/bx/8bx8 ftp://data.pdbj.org/pub/pdb/validation_reports/bx/8bx8 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  16312MC  8bwyC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 5 types, 5 molecules ACDEP
| #1: Protein | Mass: 534400.938 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila (eukaryote) / References: UniProt: Q22A67 Tetrahymena thermophila (eukaryote) / References: UniProt: Q22A67 |
|---|---|
| #3: Protein | Mass: 475554.406 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila (eukaryote) / References: UniProt: I7M6H4 Tetrahymena thermophila (eukaryote) / References: UniProt: I7M6H4 |
| #4: Protein | Mass: 76685.891 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila (eukaryote) / References: UniProt: I7M008 Tetrahymena thermophila (eukaryote) / References: UniProt: I7M008 |
| #5: Protein | Mass: 77178.062 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila (eukaryote) / References: UniProt: Q23FU1 Tetrahymena thermophila (eukaryote) / References: UniProt: Q23FU1 |
| #16: Protein |  Mass: 14256.472 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila (eukaryote) / References: UniProt: A4VD75 Tetrahymena thermophila (eukaryote) / References: UniProt: A4VD75 |
-Dynein light ... , 12 types, 12 molecules FGHIJKLMNOQR
| #6: Protein | Mass: 14751.817 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila (eukaryote) / References: UniProt: I7MHB1 Tetrahymena thermophila (eukaryote) / References: UniProt: I7MHB1 |
|---|---|
| #7: Protein | Mass: 18490.188 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila (eukaryote) / References: UniProt: Q22MV2 Tetrahymena thermophila (eukaryote) / References: UniProt: Q22MV2 |
| #8: Protein |  Mass: 10780.357 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila (eukaryote) / References: UniProt: Q1HFW2 Tetrahymena thermophila (eukaryote) / References: UniProt: Q1HFW2 |
| #9: Protein |  Mass: 12348.086 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila (eukaryote) / References: UniProt: Q1HFX0 Tetrahymena thermophila (eukaryote) / References: UniProt: Q1HFX0 |
| #10: Protein |  Mass: 11435.072 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila (eukaryote) Tetrahymena thermophila (eukaryote) |
| #11: Protein |  Mass: 10973.408 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila (eukaryote) / References: UniProt: Q1HFV9 Tetrahymena thermophila (eukaryote) / References: UniProt: Q1HFV9 |
| #12: Protein |  Mass: 12516.457 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila (eukaryote) / References: UniProt: W7XJB1 Tetrahymena thermophila (eukaryote) / References: UniProt: W7XJB1 |
| #13: Protein |  Mass: 10453.167 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila (eukaryote) / References: UniProt: Q1HFW0 Tetrahymena thermophila (eukaryote) / References: UniProt: Q1HFW0 |
| #14: Protein | Mass: 13202.817 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila (eukaryote) / References: UniProt: A4VEB3 Tetrahymena thermophila (eukaryote) / References: UniProt: A4VEB3 |
| #15: Protein | Mass: 15608.120 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila (eukaryote) / References: UniProt: Q1HGH8 Tetrahymena thermophila (eukaryote) / References: UniProt: Q1HGH8 |
| #17: Protein | Mass: 22851.654 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila (eukaryote) / References: UniProt: Q1HGH9 Tetrahymena thermophila (eukaryote) / References: UniProt: Q1HGH9 |
| #18: Protein | Mass: 17822.795 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila (eukaryote) / References: UniProt: Q1HFX4 Tetrahymena thermophila (eukaryote) / References: UniProt: Q1HFX4 |
-Antibody , 1 types, 1 molecules B
| #2: Antibody | Mass: 530182.375 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Tetrahymena thermophila (eukaryote) / References: UniProt: I7M9J2 Tetrahymena thermophila (eukaryote) / References: UniProt: I7M9J2 |
|---|
-Non-polymers , 3 types, 18 molecules 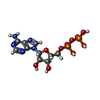
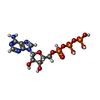



| #19: Chemical | ChemComp-ADP /  Adenosine diphosphate Adenosine diphosphate#20: Chemical |  Adenosine triphosphate Adenosine triphosphate#21: Chemical | ChemComp-MG / |
|---|
-Details
| Has ligand of interest | N |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: subtomogram averaging |
- Sample preparation
Sample preparation
| Component | Name: In situ outer dynein arm / Type: ORGANELLE OR CELLULAR COMPONENT / Entity ID: #1-#18 / Source: NATURAL |
|---|---|
| Molecular weight | Experimental value: NO |
| Source (natural) | Organism:   Chlamydomonas reinhardtii (plant) Chlamydomonas reinhardtii (plant) |
| Buffer solution | pH: 7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 4000 nm / Nominal defocus min: 3000 nm Bright-field microscopy / Nominal defocus max: 4000 nm / Nominal defocus min: 3000 nm |
| Image recording | Electron dose: 1 e/Å2 / Avg electron dose per subtomogram: 80 e/Å2 / Film or detector model: GATAN K2 QUANTUM (4k x 4k) |
- Processing
Processing
| Software | Name: UCSF ChimeraX / Version: 1.4/v9 / Classification: model building / URL: https://www.rbvi.ucsf.edu/chimerax/ / Os: Windows / Type: package |
|---|---|
CTF correction | Type: PHASE FLIPPING ONLY |
| Symmetry | Point symmetry : C1 (asymmetric) : C1 (asymmetric) |
3D reconstruction | Resolution: 30.3 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 2131 / Symmetry type: POINT |
| EM volume selection | Num. of tomograms: 8 / Num. of volumes extracted: 3167 |
| Atomic model building | Protocol: RIGID BODY FIT / Space: REAL |
 Movie
Movie Controller
Controller






 PDBj
PDBj













