[English] 日本語
 Yorodumi
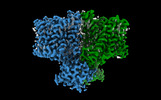
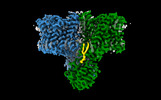
Yorodumi- PDB-7x1g: Cryo-EM structure of human BTR1 in the inward-facing state at pH 5.5 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7x1g | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human BTR1 in the inward-facing state at pH 5.5 | |||||||||
 Components Components | Isoform 1 of Solute carrier family 4 member 11 | |||||||||
 Keywords Keywords |  MEMBRANE PROTEIN / SLC4A11 / BTR1 / SLC transporter MEMBRANE PROTEIN / SLC4A11 / BTR1 / SLC transporter | |||||||||
| Function / homology |  Function and homology information Function and homology informationborate transport / active borate transmembrane transporter activity / regulation of mesenchymal stem cell differentiation / fluid transport / water transmembrane transporter activity / monoatomic ion homeostasis / intracellular monoatomic cation homeostasis / cellular hypotonic response /  proton channel activity / solute:inorganic anion antiporter activity ...borate transport / active borate transmembrane transporter activity / regulation of mesenchymal stem cell differentiation / fluid transport / water transmembrane transporter activity / monoatomic ion homeostasis / intracellular monoatomic cation homeostasis / cellular hypotonic response / proton channel activity / solute:inorganic anion antiporter activity ...borate transport / active borate transmembrane transporter activity / regulation of mesenchymal stem cell differentiation / fluid transport / water transmembrane transporter activity / monoatomic ion homeostasis / intracellular monoatomic cation homeostasis / cellular hypotonic response /  proton channel activity / solute:inorganic anion antiporter activity / proton channel activity / solute:inorganic anion antiporter activity /  symporter activity / vesicle membrane / symporter activity / vesicle membrane /  sodium channel activity / bicarbonate transport / bicarbonate transmembrane transporter activity / monoatomic anion transport / sodium ion transport / proton transmembrane transporter activity / transmembrane transporter activity / proton transmembrane transport / regulation of mitochondrial membrane potential / transmembrane transport / cellular response to oxidative stress / basolateral plasma membrane / sodium channel activity / bicarbonate transport / bicarbonate transmembrane transporter activity / monoatomic anion transport / sodium ion transport / proton transmembrane transporter activity / transmembrane transporter activity / proton transmembrane transport / regulation of mitochondrial membrane potential / transmembrane transport / cellular response to oxidative stress / basolateral plasma membrane /  protein dimerization activity / apical plasma membrane / protein dimerization activity / apical plasma membrane /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.94 Å cryo EM / Resolution: 2.94 Å | |||||||||
 Authors Authors | Yin, Y. / Lu, Y. / Zuo, P. | |||||||||
| Funding support |  China, 2items China, 2items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural insights into the conformational changes of BTR1/SLC4A11 in complex with PIP. Authors: Yishuo Lu / Peng Zuo / Hongyi Chen / Hui Shan / Weize Wang / Zonglin Dai / He Xu / Yayu Chen / Ling Liang / Dian Ding / Yan Jin / Yuxin Yin /  Abstract: BTR1 (SLC4A11) is a NH stimulated H (OH) transporter belonging to the SLC4 family. Dysfunction of BTR1 leads to diseases such as congenital hereditary endothelial dystrophy (CHED) and Fuchs ...BTR1 (SLC4A11) is a NH stimulated H (OH) transporter belonging to the SLC4 family. Dysfunction of BTR1 leads to diseases such as congenital hereditary endothelial dystrophy (CHED) and Fuchs endothelial corneal dystrophy (FECD). However, the mechanistic basis of BTR1 activation by alkaline pH, transport activity regulation and pathogenic mutations remains elusive. Here, we present cryo-EM structures of human BTR1 in the outward-facing state in complex with its activating ligands PIP and the inward-facing state with the pathogenic R125H mutation. We reveal that PIP binds at the interface between the transmembrane domain and the N-terminal cytosolic domain of BTR1. Disruption of either the PIP binding site or protonation of PIP phosphate groups by acidic pH can transform BTR1 into an inward-facing conformation. Our results provide insights into the mechanisms of how the transport activity and conformation changes of BTR1 are regulated by PIP binding and interaction of TMD and NTD. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7x1g.cif.gz 7x1g.cif.gz | 243.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7x1g.ent.gz pdb7x1g.ent.gz | 193.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7x1g.json.gz 7x1g.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/x1/7x1g https://data.pdbj.org/pub/pdb/validation_reports/x1/7x1g ftp://data.pdbj.org/pub/pdb/validation_reports/x1/7x1g ftp://data.pdbj.org/pub/pdb/validation_reports/x1/7x1g | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  32940MC  7x1hC  7x1iC  7x1jC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 99684.438 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: SLC4A11, BTR1 / Production host: Homo sapiens (human) / Gene: SLC4A11, BTR1 / Production host:   Homo sapiens (human) / References: UniProt: Q8NBS3 Homo sapiens (human) / References: UniProt: Q8NBS3 |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Solute carrier family 4 member 11, isoform B / Type: COMPLEX / Entity ID: all / Source: RECOMBINANT |
|---|---|
| Molecular weight | Value: 0.199 MDa / Experimental value: NO |
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 5.5 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 1800 nm / Nominal defocus min: 1500 nm Bright-field microscopy / Nominal defocus max: 1800 nm / Nominal defocus min: 1500 nm |
| Image recording | Electron dose: 50 e/Å2 / Detector mode: SUPER-RESOLUTION / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
- Processing
Processing
| EM software | Name: cryoSPARC / Version: 3.1.0 / Category: 3D reconstruction |
|---|---|
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
3D reconstruction | Resolution: 2.94 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 161085 / Symmetry type: POINT |
 Movie
Movie Controller
Controller



 PDBj
PDBj