+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7sp8 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Chlorella virus Hyaluronan Synthase bound to UDP-GlcNAc | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  MEMBRANE PROTEIN / MEMBRANE PROTEIN /  glycosyltransferase / glycosyltransferase /  hyaluronan hyaluronan | ||||||
| Function / homology | Glycosyltransferase like family 2 / Nucleotide-diphospho-sugar transferases /  membrane / 1,2-Distearoyl-sn-glycerophosphoethanolamine / : / URIDINE-DIPHOSPHATE-N-ACETYLGLUCOSAMINE / CHOLESTEROL HEMISUCCINATE / membrane / 1,2-Distearoyl-sn-glycerophosphoethanolamine / : / URIDINE-DIPHOSPHATE-N-ACETYLGLUCOSAMINE / CHOLESTEROL HEMISUCCINATE /  Hyaluronan synthase Hyaluronan synthase Function and homology information Function and homology information | ||||||
| Biological species |  Paramecium bursaria Chlorella virus CZ-2 Paramecium bursaria Chlorella virus CZ-2  Lama glama (llama) Lama glama (llama) | ||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.7 Å cryo EM / Resolution: 2.7 Å | ||||||
 Authors Authors | Maloney, F.P. / Kuklewicz, J. / Zimmer, J. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Structure, substrate recognition and initiation of hyaluronan synthase. Authors: Finn P Maloney / Jeremi Kuklewicz / Robin A Corey / Yunchen Bi / Ruoya Ho / Lukasz Mateusiak / Els Pardon / Jan Steyaert / Phillip J Stansfeld / Jochen Zimmer /     Abstract: Hyaluronan is an acidic heteropolysaccharide comprising alternating N-acetylglucosamine and glucuronic acid sugars that is ubiquitously expressed in the vertebrate extracellular matrix. The high- ...Hyaluronan is an acidic heteropolysaccharide comprising alternating N-acetylglucosamine and glucuronic acid sugars that is ubiquitously expressed in the vertebrate extracellular matrix. The high-molecular-mass polymer modulates essential physiological processes in health and disease, including cell differentiation, tissue homeostasis and angiogenesis. Hyaluronan is synthesized by a membrane-embedded processive glycosyltransferase, hyaluronan synthase (HAS), which catalyses the synthesis and membrane translocation of hyaluronan from uridine diphosphate-activated precursors. Here we describe five cryo-electron microscopy structures of a viral HAS homologue at different states during substrate binding and initiation of polymer synthesis. Combined with biochemical analyses and molecular dynamics simulations, our data reveal how HAS selects its substrates, hydrolyses the first substrate to prime the synthesis reaction, opens a hyaluronan-conducting transmembrane channel, ensures alternating substrate polymerization and coordinates hyaluronan inside its transmembrane pore. Our research suggests a detailed model for the formation of an acidic extracellular heteropolysaccharide and provides insights into the biosynthesis of one of the most abundant and essential glycosaminoglycans in the human body. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7sp8.cif.gz 7sp8.cif.gz | 164.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7sp8.ent.gz pdb7sp8.ent.gz | 130 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7sp8.json.gz 7sp8.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/sp/7sp8 https://data.pdbj.org/pub/pdb/validation_reports/sp/7sp8 ftp://data.pdbj.org/pub/pdb/validation_reports/sp/7sp8 ftp://data.pdbj.org/pub/pdb/validation_reports/sp/7sp8 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  25368MC  7sp6C  7sp7C  7sp9C  7spaC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
| EM raw data |  EMPIAR-11031 (Title: Cryo electron microscopy of inactive hyaluronan synthase with UDP-GlcNAc EMPIAR-11031 (Title: Cryo electron microscopy of inactive hyaluronan synthase with UDP-GlcNAcData size: 2.3 TB Data #1: Unaligned movies of Chlorella virus hyaluronan synthase [micrographs - multiframe]) |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein |  Mass: 65336.484 Da / Num. of mol.: 1 / Mutation: D302N Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Paramecium bursaria Chlorella virus CZ-2 Paramecium bursaria Chlorella virus CZ-2Gene: CZ-2_118R, PBCVCZ2_118R / Plasmid: pET28a / Production host:   Escherichia coli BL21(DE3) (bacteria) / Strain (production host): BL21(DE3) / Variant (production host): C43 / References: UniProt: M1H2Q1 Escherichia coli BL21(DE3) (bacteria) / Strain (production host): BL21(DE3) / Variant (production host): C43 / References: UniProt: M1H2Q1 |
|---|
-Antibody , 2 types, 2 molecules BC
| #2: Antibody | Mass: 14783.248 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Lama glama (llama) / Plasmid: pMESy4 / Production host: Lama glama (llama) / Plasmid: pMESy4 / Production host:   Escherichia coli K-12 (bacteria) / Strain (production host): WK6 Escherichia coli K-12 (bacteria) / Strain (production host): WK6 |
|---|---|
| #3: Antibody | Mass: 15265.755 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Lama glama (llama) / Plasmid: pMESy4 / Production host: Lama glama (llama) / Plasmid: pMESy4 / Production host:   Escherichia coli K-12 (bacteria) Escherichia coli K-12 (bacteria) |
-Non-polymers , 4 types, 5 molecules 

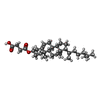




| #4: Chemical | ChemComp-UD1 / |
|---|---|
| #5: Chemical | ChemComp-3PE /  Phosphatidylethanolamine Phosphatidylethanolamine |
| #6: Chemical | ChemComp-Y01 / |
| #7: Chemical |
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Hyaluronan synthase in nanodiscs in complex with two camelid nanobodies. Type: COMPLEX / Entity ID: #1-#3 / Source: MULTIPLE SOURCES | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.0958 MDa / Experimental value: NO | ||||||||||||||||||||||||||||||
| Source (natural) |
| ||||||||||||||||||||||||||||||
| Source (recombinant) |
| ||||||||||||||||||||||||||||||
| Buffer solution | pH: 7.5 | ||||||||||||||||||||||||||||||
| Buffer component |
| ||||||||||||||||||||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES | ||||||||||||||||||||||||||||||
| Specimen support | Details: Sample incubated on grid 30s before blotting. Blot 4 seconds with force 4. Grid material: COPPER / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R1.2/1.3 | ||||||||||||||||||||||||||||||
Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277.15 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal magnification: 81000 X / Nominal defocus max: 2000 nm / Nominal defocus min: 1000 nm / Cs Bright-field microscopy / Nominal magnification: 81000 X / Nominal defocus max: 2000 nm / Nominal defocus min: 1000 nm / Cs : 2.7 mm : 2.7 mm |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Electron dose: 51 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) / Num. of grids imaged: 1 / Num. of real images: 8218 / Details: 40-frame movies were collected |
| EM imaging optics | Energyfilter name : GIF Bioquantum / Energyfilter slit width: 10 eV : GIF Bioquantum / Energyfilter slit width: 10 eV |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 4391341 | ||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry : C1 (asymmetric) : C1 (asymmetric) | ||||||||||||||||||||||||||||||||
3D reconstruction | Resolution: 2.7 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 105056 / Num. of class averages: 1 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||
| Refinement | Cross valid method: NONE Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2 | ||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 66.7 Å2 | ||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller







 PDBj
PDBj







