Entry Database : PDB / ID : 5zzaTitle OdinProfilin/Rabbit Actin Complex Actin, alpha skeletal muscle Uncharacterized protein Keywords / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Candidatus Odinarchaeota archaeon LCB_4 (archaea)Oryctolagus cuniculus (rabbit)Method / / / Resolution : 1.53 Å Authors Robinson, R.C. / Akil, C. Journal : Nature / Year : 2018Title : Genomes of Asgard archaea encode profilins that regulate actin.Authors : Akil, C. / Robinson, R.C. History Deposition May 31, 2018 Deposition site / Processing site Revision 1.0 Oct 10, 2018 Provider / Type Revision 1.1 Oct 17, 2018 Group / Database references / Structure summaryCategory / citation_author / entityItem / _citation.title / _entity.formula_weightRevision 1.2 Oct 31, 2018 Group / Database references / Category / citation_author / diffrn_detectorItem _citation.journal_volume / _citation.page_first ... _citation.journal_volume / _citation.page_first / _citation.page_last / _diffrn_detector.detector / _diffrn_detector.type Revision 1.3 Dec 5, 2018 Group / Database references / Category Revision 1.4 Nov 22, 2023 Group Data collection / Database references ... Data collection / Database references / Derived calculations / Refinement description Category chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model / pdbx_struct_conn_angle / refine_hist / struct_conn Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_auth_seq_id / _pdbx_struct_conn_angle.value / _refine_hist.d_res_low / _struct_conn.pdbx_dist_value / _struct_conn.ptnr2_auth_seq_id
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords STRUCTURAL PROTEIN /
STRUCTURAL PROTEIN /  Actin /
Actin /  Archaea / Odin
Archaea / Odin Function and homology information
Function and homology information tropomyosin binding /
tropomyosin binding /  myosin heavy chain binding / mesenchyme migration /
myosin heavy chain binding / mesenchyme migration /  troponin I binding / actin filament bundle / filamentous actin / actin filament bundle assembly / skeletal muscle thin filament assembly / striated muscle thin filament ...cytoskeletal motor activator activity /
troponin I binding / actin filament bundle / filamentous actin / actin filament bundle assembly / skeletal muscle thin filament assembly / striated muscle thin filament ...cytoskeletal motor activator activity /  tropomyosin binding /
tropomyosin binding /  myosin heavy chain binding / mesenchyme migration /
myosin heavy chain binding / mesenchyme migration /  troponin I binding / actin filament bundle / filamentous actin / actin filament bundle assembly / skeletal muscle thin filament assembly / striated muscle thin filament / skeletal muscle myofibril / actin monomer binding / skeletal muscle fiber development /
troponin I binding / actin filament bundle / filamentous actin / actin filament bundle assembly / skeletal muscle thin filament assembly / striated muscle thin filament / skeletal muscle myofibril / actin monomer binding / skeletal muscle fiber development /  stress fiber /
stress fiber /  titin binding / actin filament polymerization /
titin binding / actin filament polymerization /  filopodium /
filopodium /  actin filament /
actin filament /  Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / calcium-dependent protein binding /
Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / calcium-dependent protein binding /  lamellipodium /
lamellipodium /  cell body /
cell body /  actin binding /
actin binding /  cytoskeleton /
cytoskeleton /  hydrolase activity / protein domain specific binding /
hydrolase activity / protein domain specific binding /  calcium ion binding / positive regulation of gene expression / magnesium ion binding /
calcium ion binding / positive regulation of gene expression / magnesium ion binding /  ATP binding / identical protein binding /
ATP binding / identical protein binding /  cytoplasm
cytoplasm Candidatus Odinarchaeota archaeon LCB_4 (archaea)
Candidatus Odinarchaeota archaeon LCB_4 (archaea)
 Oryctolagus cuniculus (rabbit)
Oryctolagus cuniculus (rabbit) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.53 Å
MOLECULAR REPLACEMENT / Resolution: 1.53 Å  Authors
Authors Citation
Citation Journal: Nature / Year: 2018
Journal: Nature / Year: 2018 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5zza.cif.gz
5zza.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5zza.ent.gz
pdb5zza.ent.gz PDB format
PDB format 5zza.json.gz
5zza.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/zz/5zza
https://data.pdbj.org/pub/pdb/validation_reports/zz/5zza ftp://data.pdbj.org/pub/pdb/validation_reports/zz/5zza
ftp://data.pdbj.org/pub/pdb/validation_reports/zz/5zza


 Links
Links Assembly
Assembly
 Components
Components Candidatus Odinarchaeota archaeon LCB_4 (archaea)
Candidatus Odinarchaeota archaeon LCB_4 (archaea)
 Escherichia coli (E. coli) / References: UniProt: A0A1Q9N7W7
Escherichia coli (E. coli) / References: UniProt: A0A1Q9N7W7 / Alpha-actin-1
/ Alpha-actin-1
 Oryctolagus cuniculus (rabbit) / References: UniProt: P68135
Oryctolagus cuniculus (rabbit) / References: UniProt: P68135
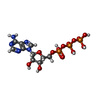





 Latrunculin
Latrunculin Adenosine triphosphate
Adenosine triphosphate Water
Water X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation
 SYNCHROTRON / Site:
SYNCHROTRON / Site:  Australian Synchrotron
Australian Synchrotron  / Beamline: MX2 / Wavelength: 1 Å
/ Beamline: MX2 / Wavelength: 1 Å : 1 Å / Relative weight: 1
: 1 Å / Relative weight: 1  Processing
Processing :
:  MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller













 PDBj
PDBj




