[English] 日本語
 Yorodumi
Yorodumi- PDB-4gkh: Crystal structure of the aminoglycoside phosphotransferase APH(3'... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4gkh | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the aminoglycoside phosphotransferase APH(3')-Ia, with substrate kanamycin and small molecule inhibitor 1-NA-PP1 | ||||||
 Components Components | Aminoglycoside 3'-phosphotransferase AphA1-IAB | ||||||
 Keywords Keywords | TRANSFERASE/TRANSFERASE INHIBITOR /  PYRAZOLOPYRIMIDINE / 1-NA-PP1 / BUMPED KINASE INHIBITOR / BKI / PYRAZOLOPYRIMIDINE / 1-NA-PP1 / BUMPED KINASE INHIBITOR / BKI /  PROTEIN KINASE INHIBITOR / CENTER FOR STRUCTURAL GENOMICS OF INFECTIOUS DISEASES / CSGID / NIAID / PROTEIN KINASE INHIBITOR / CENTER FOR STRUCTURAL GENOMICS OF INFECTIOUS DISEASES / CSGID / NIAID /  NATIONAL INSTITUTE OF ALLERGY AND INFECTIOUS DISEASES / TRANSFERASE-TRANSFERASE INHIBITOR COMPLEX / NATIONAL INSTITUTE OF ALLERGY AND INFECTIOUS DISEASES / TRANSFERASE-TRANSFERASE INHIBITOR COMPLEX /  EUKARYOTIC PROTEIN KINASE-LIKE FOLD / ALPHA/BETA PROTEIN / EUKARYOTIC PROTEIN KINASE-LIKE FOLD / ALPHA/BETA PROTEIN /  TRANSFERASE / TRANSFERASE /  PHOSPHOTRANSFERASE / AMINOGLYCOSIDE PHOSPHOTRANSFERASE / PHOSPHOTRANSFERASE / AMINOGLYCOSIDE PHOSPHOTRANSFERASE /  ANTIBIOTIC RESISTANCE / ANTIBIOTIC RESISTANCE /  AMINOGLYCOSIDES / AMINOGLYCOSIDES /  KANAMYCIN / GTP / KANAMYCIN / GTP /  INTRACELLULAR INTRACELLULAR | ||||||
| Function / homology |  Function and homology information Function and homology information kanamycin kinase / kanamycin kinase /  kanamycin kinase activity / kanamycin kinase activity /  phosphorylation / response to antibiotic / phosphorylation / response to antibiotic /  ATP binding / ATP binding /  metal ion binding metal ion bindingSimilarity search - Function | ||||||
| Biological species |   Acinetobacter baumannii (bacteria) Acinetobacter baumannii (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.863 Å MOLECULAR REPLACEMENT / Resolution: 1.863 Å | ||||||
 Authors Authors | Stogios, P.J. / Evdokimova, E. / Wawrzak, Z. / Minasov, G. / Egorova, O. / Di Leo, R. / Shakya, T. / Spanogiannopoulos, P. / Todorovic, N. / Capretta, A. ...Stogios, P.J. / Evdokimova, E. / Wawrzak, Z. / Minasov, G. / Egorova, O. / Di Leo, R. / Shakya, T. / Spanogiannopoulos, P. / Todorovic, N. / Capretta, A. / Wright, G.D. / Savchenko, A. / Anderson, W.F. / Center for Structural Genomics of Infectious Diseases (CSGID) | ||||||
 Citation Citation |  Journal: Biochem.J. / Year: 2013 Journal: Biochem.J. / Year: 2013Title: Structure-guided optimization of protein kinase inhibitors reverses aminoglycoside antibiotic resistance. Authors: Stogios, P.J. / Spanogiannopoulos, P. / Evdokimova, E. / Egorova, O. / Shakya, T. / Todorovic, N. / Capretta, A. / Wright, G.D. / Savchenko, A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4gkh.cif.gz 4gkh.cif.gz | 1.3 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4gkh.ent.gz pdb4gkh.ent.gz | 1.1 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4gkh.json.gz 4gkh.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gk/4gkh https://data.pdbj.org/pub/pdb/validation_reports/gk/4gkh ftp://data.pdbj.org/pub/pdb/validation_reports/gk/4gkh ftp://data.pdbj.org/pub/pdb/validation_reports/gk/4gkh | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4ej7SC  4feuC  4fevC  4fewC  4fexC  4gkiC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | |
| Other databases |
- Links
Links
- Assembly
Assembly
- Components
Components
-Protein , 1 types, 12 molecules ABCDEFGHIJKL
| #1: Protein | Mass: 31350.404 Da / Num. of mol.: 12 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Acinetobacter baumannii (bacteria) / Strain: AYE / Gene: ABAYE3578, APHA1-IAB / Plasmid: P15TV LIC / Production host: Acinetobacter baumannii (bacteria) / Strain: AYE / Gene: ABAYE3578, APHA1-IAB / Plasmid: P15TV LIC / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: B0VD92, Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: B0VD92,  kanamycin kinase kanamycin kinase |
|---|
-Non-polymers , 7 types, 2949 molecules 


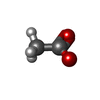









| #2: Chemical | ChemComp-KAN /  Kanamycin A Kanamycin A#3: Chemical | ChemComp-0J9 / #4: Chemical | ChemComp-NA / #5: Chemical | ChemComp-ACT /  Acetate Acetate#6: Chemical |  Chloride Chloride#7: Chemical |  Diethylene glycol Diethylene glycol#8: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.33 Å3/Da / Density % sol: 47.17 % |
|---|---|
Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 4.5 Details: 0.1 M SODIUM ACETATE pH 4.5, 7% PEG3350, 3% DMSO, 2 MM KANAMYCIN, 3 mM 1-NA-PP1, VAPOR DIFFUSION, HANGING DROP, temperature 298K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 21-ID-G / Wavelength: 0.97856 Å / Beamline: 21-ID-G / Wavelength: 0.97856 Å |
| Detector | Type: MARMOSAIC 300 mm CCD / Detector: CCD / Date: Apr 21, 2012 |
| Radiation | Monochromator: DIAMOND(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.97856 Å / Relative weight: 1 : 0.97856 Å / Relative weight: 1 |
| Reflection | Resolution: 1.86→80.87 Å / Num. obs: 275562 / % possible obs: 97.2 % / Observed criterion σ(F): 0 / Observed criterion σ(I): -2 / Redundancy: 3.9 % / Rmerge(I) obs: 0.061 / Net I/σ(I): 11.1 |
| Reflection shell | Resolution: 1.86→1.96 Å / Redundancy: 3.9 % / Rmerge(I) obs: 0.401 / Mean I/σ(I) obs: 2.8 / % possible all: 96.5 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4EJ7 Resolution: 1.863→54.779 Å / SU ML: 0.19 / σ(F): 1.96 / Phase error: 23.78 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.863→54.779 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj
























