[English] 日本語
 Yorodumi
Yorodumi- PDB-3q4t: Crystal structure of Activin receptor type-IIA (ACVR2A) kinase do... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3q4t | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of Activin receptor type-IIA (ACVR2A) kinase domain in complex with dorsomorphin | ||||||
 Components Components | Activin receptor type-2A | ||||||
 Keywords Keywords |  TRANSFERASE / TRANSFERASE /  Structural Genomics Consortium / SGC / Structural Genomics Consortium / SGC /  Protein kinase Protein kinase | ||||||
| Function / homology |  Function and homology information Function and homology informationRegulation of signaling by NODAL / inhibin-betaglycan-ActRII complex /  inhibin binding / inhibin binding /  penile erection / positive regulation of activin receptor signaling pathway / penile erection / positive regulation of activin receptor signaling pathway /  activin receptor activity / Sertoli cell proliferation / sperm ejaculation / BMP receptor activity / embryonic skeletal system development ...Regulation of signaling by NODAL / inhibin-betaglycan-ActRII complex / activin receptor activity / Sertoli cell proliferation / sperm ejaculation / BMP receptor activity / embryonic skeletal system development ...Regulation of signaling by NODAL / inhibin-betaglycan-ActRII complex /  inhibin binding / inhibin binding /  penile erection / positive regulation of activin receptor signaling pathway / penile erection / positive regulation of activin receptor signaling pathway /  activin receptor activity / Sertoli cell proliferation / sperm ejaculation / BMP receptor activity / embryonic skeletal system development / activin receptor activity / Sertoli cell proliferation / sperm ejaculation / BMP receptor activity / embryonic skeletal system development /  activin receptor complex / activin receptor complex /  receptor protein serine/threonine kinase / receptor protein serine/threonine kinase /  transmembrane receptor protein serine/threonine kinase activity / Signaling by BMP / transmembrane receptor protein serine/threonine kinase activity / Signaling by BMP /  activin binding / cellular response to BMP stimulus / activin receptor signaling pathway / Signaling by Activin / Signaling by NODAL / gastrulation with mouth forming second / regulation of nitric oxide biosynthetic process / determination of left/right symmetry / anterior/posterior pattern specification / activin binding / cellular response to BMP stimulus / activin receptor signaling pathway / Signaling by Activin / Signaling by NODAL / gastrulation with mouth forming second / regulation of nitric oxide biosynthetic process / determination of left/right symmetry / anterior/posterior pattern specification /  cell surface receptor protein serine/threonine kinase signaling pathway / odontogenesis of dentin-containing tooth / cell surface receptor protein serine/threonine kinase signaling pathway / odontogenesis of dentin-containing tooth /  growth factor binding / mesoderm development / positive regulation of SMAD protein signal transduction / BMP signaling pathway / positive regulation of bone mineralization / positive regulation of osteoblast differentiation / growth factor binding / mesoderm development / positive regulation of SMAD protein signal transduction / BMP signaling pathway / positive regulation of bone mineralization / positive regulation of osteoblast differentiation /  coreceptor activity / positive regulation of erythrocyte differentiation / coreceptor activity / positive regulation of erythrocyte differentiation /  PDZ domain binding / cellular response to growth factor stimulus / : / PDZ domain binding / cellular response to growth factor stimulus / : /  spermatogenesis / spermatogenesis /  receptor complex / positive regulation of protein phosphorylation / receptor complex / positive regulation of protein phosphorylation /  phosphorylation / protein serine/threonine kinase activity / phosphorylation / protein serine/threonine kinase activity /  cell surface / positive regulation of transcription by RNA polymerase II / cell surface / positive regulation of transcription by RNA polymerase II /  ATP binding / ATP binding /  metal ion binding / metal ion binding /  plasma membrane / plasma membrane /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.96 Å MOLECULAR REPLACEMENT / Resolution: 1.96 Å | ||||||
 Authors Authors | Chaikuad, A. / Alfano, I. / Mahajan, P. / Cooper, C.D.O. / Sanvitale, C. / Vollmar, M. / Krojer, T. / Muniz, J.R.C. / Raynor, J. / von Delft, F. ...Chaikuad, A. / Alfano, I. / Mahajan, P. / Cooper, C.D.O. / Sanvitale, C. / Vollmar, M. / Krojer, T. / Muniz, J.R.C. / Raynor, J. / von Delft, F. / Weigelt, J. / Arrowsmith, C.H. / Edwards, A.M. / Bountra, C. / Bullock, A. / Structural Genomics Consortium (SGC) | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2015 Journal: J.Biol.Chem. / Year: 2015Title: Small Molecules Dorsomorphin and LDN-193189 Inhibit Myostatin/GDF8 Signaling and Promote Functional Myoblast Differentiation. Authors: Horbelt, D. / Boergermann, J.H. / Chaikuad, A. / Alfano, I. / Williams, E. / Lukonin, I. / Timmel, T. / Bullock, A.N. / Knaus, P. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3q4t.cif.gz 3q4t.cif.gz | 270.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3q4t.ent.gz pdb3q4t.ent.gz | 218.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3q4t.json.gz 3q4t.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/q4/3q4t https://data.pdbj.org/pub/pdb/validation_reports/q4/3q4t ftp://data.pdbj.org/pub/pdb/validation_reports/q4/3q4t ftp://data.pdbj.org/pub/pdb/validation_reports/q4/3q4t | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2qluS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 36548.766 Da / Num. of mol.: 2 / Fragment: kinase domain (UNP residues 191-488) Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: ACVR2, ACVR2A / Plasmid: pFB-LIC-Bse / Cell line (production host): SF9 / Production host: Homo sapiens (human) / Gene: ACVR2, ACVR2A / Plasmid: pFB-LIC-Bse / Cell line (production host): SF9 / Production host:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm)References: UniProt: P27037,  receptor protein serine/threonine kinase receptor protein serine/threonine kinase |
|---|
-Non-polymers , 5 types, 524 molecules 

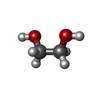
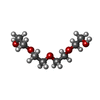





| #2: Chemical | | #3: Chemical | ChemComp-SO4 /  Sulfate Sulfate#4: Chemical | ChemComp-EDO /  Ethylene glycol Ethylene glycol#5: Chemical | ChemComp-PG4 / |  Polyethylene glycol Polyethylene glycol#6: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.48 Å3/Da / Density % sol: 50.33 % |
|---|---|
Crystal grow | Temperature: 293.15 K / Method: vapor diffusion, sitting drop / pH: 8.8 Details: 28% PEG 3350, 0.2M LiSO4, 0.1M Tris, pH 8.8, 10% Ethylene glycol, VAPOR DIFFUSION, SITTING DROP, temperature 293.15K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I03 / Wavelength: 0.9763 Å / Beamline: I03 / Wavelength: 0.9763 Å |
| Detector | Type: ADSC Q315 3x3 CCD / Detector: CCD / Date: Apr 21, 2010 / Details: Kirkpatrick Baez bimorph mirror pair |
| Radiation | Monochromator: Si (111) double crystal monochromator / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.9763 Å / Relative weight: 1 : 0.9763 Å / Relative weight: 1 |
| Reflection | Resolution: 1.96→48.6 Å / Num. all: 53883 / Num. obs: 53760 / % possible obs: 99.9 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 7 % / Biso Wilson estimate: 26.6 Å2 / Rmerge(I) obs: 0.112 / Net I/σ(I): 11.2 |
| Reflection shell | Resolution: 1.96→2.07 Å / Redundancy: 7.2 % / Rmerge(I) obs: 0.783 / Mean I/σ(I) obs: 2.3 / Num. unique all: 7727 / % possible all: 100 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: pdb id 2qlu Resolution: 1.96→45.47 Å / Cor.coef. Fo:Fc: 0.963 / Cor.coef. Fo:Fc free: 0.939 / SU B: 6.75 / SU ML: 0.1 / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 2 / ESU R Free: 0.147 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN USED IN REFINEMENT BUT NOT OUTPUT TO PDB
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 24.429 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.209 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.96→45.47 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.96→2.011 Å / Total num. of bins used: 20
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj





