[English] 日本語
 Yorodumi
Yorodumi- PDB-2v7q: The structure of F1-ATPase inhibited by I1-60HIS, a monomeric for... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2v7q | ||||||
|---|---|---|---|---|---|---|---|
| Title | The structure of F1-ATPase inhibited by I1-60HIS, a monomeric form of the inhibitor protein, IF1. | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  HYDROLASE / HYDROLASE /  ION TRANSPORT / ION TRANSPORT /  MITOCHONDRION / TRANSIT PEPTIDE / MITOCHONDRION / TRANSIT PEPTIDE /  INHIBITOR PROTEIN INHIBITOR PROTEIN | ||||||
| Function / homology |  Function and homology information Function and homology information angiostatin binding / Formation of ATP by chemiosmotic coupling / Cristae formation / ATPase inhibitor activity / mitochondrial envelope / mitochondrial proton-transporting ATP synthase complex assembly / negative regulation of hydrolase activity / proton-transporting ATP synthase complex / heme biosynthetic process / angiostatin binding / Formation of ATP by chemiosmotic coupling / Cristae formation / ATPase inhibitor activity / mitochondrial envelope / mitochondrial proton-transporting ATP synthase complex assembly / negative regulation of hydrolase activity / proton-transporting ATP synthase complex / heme biosynthetic process /  Mitochondrial protein degradation ... Mitochondrial protein degradation ... angiostatin binding / Formation of ATP by chemiosmotic coupling / Cristae formation / ATPase inhibitor activity / mitochondrial envelope / mitochondrial proton-transporting ATP synthase complex assembly / negative regulation of hydrolase activity / proton-transporting ATP synthase complex / heme biosynthetic process / angiostatin binding / Formation of ATP by chemiosmotic coupling / Cristae formation / ATPase inhibitor activity / mitochondrial envelope / mitochondrial proton-transporting ATP synthase complex assembly / negative regulation of hydrolase activity / proton-transporting ATP synthase complex / heme biosynthetic process /  Mitochondrial protein degradation / mitochondrial proton-transporting ATP synthase, catalytic core / mitochondrial proton-transporting ATP synthase complex, catalytic sector F(1) / mitochondrial proton-transporting ATP synthase complex / negative regulation of endothelial cell proliferation / proton motive force-driven ATP synthesis / proton transmembrane transporter activity / proton motive force-driven mitochondrial ATP synthesis / proton-transporting ATP synthase complex, catalytic core F(1) / Mitochondrial protein degradation / mitochondrial proton-transporting ATP synthase, catalytic core / mitochondrial proton-transporting ATP synthase complex, catalytic sector F(1) / mitochondrial proton-transporting ATP synthase complex / negative regulation of endothelial cell proliferation / proton motive force-driven ATP synthesis / proton transmembrane transporter activity / proton motive force-driven mitochondrial ATP synthesis / proton-transporting ATP synthase complex, catalytic core F(1) /  H+-transporting two-sector ATPase / H+-transporting two-sector ATPase /  aerobic respiration / proton transmembrane transport / proton-transporting ATPase activity, rotational mechanism / proton-transporting ATP synthase activity, rotational mechanism / aerobic respiration / proton transmembrane transport / proton-transporting ATPase activity, rotational mechanism / proton-transporting ATP synthase activity, rotational mechanism /  erythrocyte differentiation / erythrocyte differentiation /  ADP binding / ADP binding /  ATPase binding / protein homotetramerization / ATPase binding / protein homotetramerization /  mitochondrial inner membrane / mitochondrial inner membrane /  calmodulin binding / structural molecule activity / calmodulin binding / structural molecule activity /  cell surface / protein homodimerization activity / cell surface / protein homodimerization activity /  ATP hydrolysis activity / protein-containing complex / ATP hydrolysis activity / protein-containing complex /  mitochondrion / mitochondrion /  ATP binding / identical protein binding / ATP binding / identical protein binding /  plasma membrane plasma membraneSimilarity search - Function | ||||||
| Biological species |   BOS TAURUS (cattle) BOS TAURUS (cattle) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.1 Å MOLECULAR REPLACEMENT / Resolution: 2.1 Å | ||||||
 Authors Authors | Gledhill, J.R. / Montgomery, M.G. / Leslie, A.G.W. / Walker, J.E. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2007 Journal: Proc.Natl.Acad.Sci.USA / Year: 2007Title: How the Regulatory Protein, If1, Inhibits F1- ATPase from Bovine Mitochondria. Authors: Gledhill, J.R. / Montgomery, M.G. / Leslie, A.G.W. / Walker, J.E. | ||||||
| History |
| ||||||
| Remark 700 | SHEET DETERMINATION METHOD: DSSP THE SHEETS PRESENTED AS "AA" IN EACH CHAIN ON SHEET RECORDS BELOW ... SHEET DETERMINATION METHOD: DSSP THE SHEETS PRESENTED AS "AA" IN EACH CHAIN ON SHEET RECORDS BELOW IS ACTUALLY AN 13-STRANDED BARREL THIS IS REPRESENTED BY A 14-STRANDED SHEET IN WHICH THE FIRST AND LAST STRANDS ARE IDENTICAL. THE SHEETS PRESENTED AS "BA" IN EACH CHAIN ON SHEET RECORDS BELOW IS ACTUALLY AN 11-STRANDED BARREL THIS IS REPRESENTED BY A 12-STRANDED SHEET IN WHICH THE FIRST AND LAST STRANDS ARE IDENTICAL. THE SHEETS PRESENTED AS "CA" IN EACH CHAIN ON SHEET RECORDS BELOW IS ACTUALLY AN 11-STRANDED BARREL THIS IS REPRESENTED BY A 12-STRANDED SHEET IN WHICH THE FIRST AND LAST STRANDS ARE IDENTICAL. THE SHEET STRUCTURE OF THIS MOLECULE IS BIFURCATED. IN ORDER TO REPRESENT THIS FEATURE IN THE SHEET RECORDS BELOW, TWO SHEETS ARE DEFINED. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2v7q.cif.gz 2v7q.cif.gz | 679.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2v7q.ent.gz pdb2v7q.ent.gz | 552.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2v7q.json.gz 2v7q.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/v7/2v7q https://data.pdbj.org/pub/pdb/validation_reports/v7/2v7q ftp://data.pdbj.org/pub/pdb/validation_reports/v7/2v7q ftp://data.pdbj.org/pub/pdb/validation_reports/v7/2v7q | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1ohhS  2uys S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-ATP SYNTHASE ... , 5 types, 9 molecules ABCDEFGHI
| #1: Protein |  / ATP SYNTHASE ALPHA CHAIN HEART ISOFORM / ATP SYNTHASE ALPHA CHAIN HEART ISOFORMMass: 55332.219 Da / Num. of mol.: 3 / Fragment: RESIDUES 44-553 / Source method: isolated from a natural source / Source: (natural)   BOS TAURUS (cattle) / Organ: HEART BOS TAURUS (cattle) / Organ: HEART / Tissue: MUSCLE / Tissue: MUSCLE Skeletal muscle Skeletal muscleReferences: UniProt: Q1JQC4, UniProt: P19483*PLUS, EC: 3.6.1.14 #2: Protein |  / ATP SYNTHASE BETA CHAIN / ATP SYNTHASE BETA CHAINMass: 51757.836 Da / Num. of mol.: 3 / Fragment: RESIDUES 47-528 / Source method: isolated from a natural source / Source: (natural)   BOS TAURUS (cattle) / Organ: HEART BOS TAURUS (cattle) / Organ: HEART / Tissue: MUSCLE / Tissue: MUSCLE Skeletal muscle / References: UniProt: P00829, EC: 3.6.1.14 Skeletal muscle / References: UniProt: P00829, EC: 3.6.1.14#3: Protein | | Mass: 30185.674 Da / Num. of mol.: 1 / Fragment: RESIDUES 26-297 / Source method: isolated from a natural source / Source: (natural)   BOS TAURUS (cattle) / Organ: HEART BOS TAURUS (cattle) / Organ: HEART / Tissue: MUSCLE / Tissue: MUSCLE Skeletal muscle / References: UniProt: P05631, EC: 3.6.1.34 Skeletal muscle / References: UniProt: P05631, EC: 3.6.1.34#4: Protein | | Mass: 15074.813 Da / Num. of mol.: 1 / Fragment: RESIDUES 23-168 / Source method: isolated from a natural source / Source: (natural)   BOS TAURUS (cattle) / Organ: HEART BOS TAURUS (cattle) / Organ: HEART / Tissue: MUSCLE / Tissue: MUSCLE Skeletal muscle / References: UniProt: P05630, EC: 3.6.1.14 Skeletal muscle / References: UniProt: P05630, EC: 3.6.1.14#5: Protein/peptide | | Mass: 5662.693 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   BOS TAURUS (cattle) / Organ: HEART BOS TAURUS (cattle) / Organ: HEART / Tissue: MUSCLE / Tissue: MUSCLE Skeletal muscle / References: UniProt: P05632, EC: 3.6.1.14 Skeletal muscle / References: UniProt: P05632, EC: 3.6.1.14 |
|---|
-Protein , 1 types, 1 molecules J
| #6: Protein | Mass: 7462.098 Da / Num. of mol.: 1 / Fragment: RESIDUES 26-85 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   BOS TAURUS (cattle) / Tissue: MUSCLE BOS TAURUS (cattle) / Tissue: MUSCLE Skeletal muscle / Organ: HEART Skeletal muscle / Organ: HEART / Production host: / Production host:   ESCHERICHIA COLI (E. coli) / Strain (production host): BL21 (DE3) / References: UniProt: P01096 ESCHERICHIA COLI (E. coli) / Strain (production host): BL21 (DE3) / References: UniProt: P01096 |
|---|
-Non-polymers , 5 types, 1951 molecules 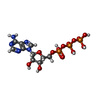






| #7: Chemical |  Adenosine triphosphate Adenosine triphosphate#8: Chemical | ChemComp-MG / #9: Chemical |  Adenosine diphosphate Adenosine diphosphate#10: Chemical | ChemComp-PO4 / |  Phosphate Phosphate#11: Water | ChemComp-HOH / |  Water Water |
|---|
-Details
| Sequence details | I1-60HIS IS A TRUNCATED FRAGMENT OF THE BOVINE ATPASE INHIBITOR PROTEIN. RESIDUES 1-60 PLUS 6 C- ...I1-60HIS IS A TRUNCATED FRAGMENT OF THE BOVINE ATPASE INHIBITOR PROTEIN. RESIDUES 1-60 PLUS 6 C-TERMINAL HISTIDINES |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.39 Å3/Da / Density % sol: 48.21 % / Description: NONE |
|---|---|
Crystal grow | pH: 8.2 / Details: pH 8.2 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID29 / Wavelength: 0.979 / Beamline: ID29 / Wavelength: 0.979 |
| Detector | Type: ADSC CCD / Detector: CCD |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.979 Å / Relative weight: 1 : 0.979 Å / Relative weight: 1 |
| Reflection | Resolution: 2.1→69.7 Å / Num. obs: 212416 / % possible obs: 99.2 % / Redundancy: 3.5 % / Rmerge(I) obs: 0.11 / Net I/σ(I): 9.1 |
| Reflection shell | Resolution: 2.1→2.21 Å / Redundancy: 3.1 % / Rmerge(I) obs: 0.42 / Mean I/σ(I) obs: 2 / % possible all: 97.7 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1OHH Resolution: 2.1→37.19 Å / Cor.coef. Fo:Fc: 0.949 / Cor.coef. Fo:Fc free: 0.916 / SU B: 10.233 / SU ML: 0.144 / TLS residual ADP flag: LIKELY RESIDUAL / Cross valid method: THROUGHOUT / ESU R: 0.223 / ESU R Free: 0.193 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: BABINET MODEL WITH MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 41.41 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.1→37.19 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller





























 PDBj
PDBj





