Entry Database : PDB / ID : 2ovzTitle MMP-9 active site mutant with phosphinate inhibitor Matrix metalloproteinase-9 (EC 3.4.24.35) (MMP-9) (92 kDa type IV collagenase) (92 kDa gelatinase) (Gelatinase B) (GELB) Keywords / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Method / / / Resolution : 2 Å Authors Tochowicz, A. / Bode, W. / Maskos, K. / Goettig, P. Journal : J.Mol.Biol. / Year : 2007Title : Crystal Structures of MMP-9 Complexes with Five Inhibitors: Contribution of the Flexible Arg424 Side-chain to Selectivity.Authors : Tochowicz, A. / Maskos, K. / Huber, R. / Oltenfreiter, R. / Dive, V. / Yiotakis, A. / Zanda, M. / Bode, W. / Goettig, P. History Deposition Feb 15, 2007 Deposition site / Processing site Revision 1.0 Jun 19, 2007 Provider / Type Revision 1.1 May 1, 2008 Group Revision 1.2 Jul 13, 2011 Group / Version format complianceRevision 1.3 Aug 16, 2017 Group / Source and taxonomyCategory / pdbx_unobs_or_zero_occ_atoms / pdbx_unobs_or_zero_occ_residuesRevision 1.4 Oct 20, 2021 Group / Database references / Derived calculationsCategory database_2 / pdbx_struct_conn_angle ... database_2 / pdbx_struct_conn_angle / pdbx_unobs_or_zero_occ_atoms / pdbx_unobs_or_zero_occ_residues / struct_conn / struct_ref_seq_dif / struct_site Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_struct_conn_angle.ptnr1_auth_comp_id / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr1_label_asym_id / _pdbx_struct_conn_angle.ptnr1_label_atom_id / _pdbx_struct_conn_angle.ptnr1_label_comp_id / _pdbx_struct_conn_angle.ptnr1_label_seq_id / _pdbx_struct_conn_angle.ptnr2_auth_comp_id / _pdbx_struct_conn_angle.ptnr2_auth_seq_id / _pdbx_struct_conn_angle.ptnr2_label_asym_id / _pdbx_struct_conn_angle.ptnr2_label_atom_id / _pdbx_struct_conn_angle.ptnr2_label_comp_id / _pdbx_struct_conn_angle.ptnr3_auth_comp_id / _pdbx_struct_conn_angle.ptnr3_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_label_asym_id / _pdbx_struct_conn_angle.ptnr3_label_atom_id / _pdbx_struct_conn_angle.ptnr3_label_comp_id / _pdbx_struct_conn_angle.ptnr3_label_seq_id / _pdbx_struct_conn_angle.value / _struct_conn.pdbx_dist_value / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_label_seq_id / _struct_ref_seq_dif.details / _struct_site.pdbx_auth_asym_id / _struct_site.pdbx_auth_comp_id / _struct_site.pdbx_auth_seq_id Revision 1.5 Aug 30, 2023 Group / Refinement descriptionCategory / chem_comp_bond / pdbx_initial_refinement_model
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords MATRIX METALLOPROTEINASE / S1-PRIME POCKET / HYDROLASE-HYDROLASE INHIBITOR complex
MATRIX METALLOPROTEINASE / S1-PRIME POCKET / HYDROLASE-HYDROLASE INHIBITOR complex Function and homology information
Function and homology information gelatinase B / negative regulation of epithelial cell differentiation involved in kidney development / negative regulation of cation channel activity / negative regulation of cysteine-type endopeptidase activity involved in apoptotic signaling pathway / cellular response to UV-A / positive regulation of keratinocyte migration / regulation of neuroinflammatory response / positive regulation of epidermal growth factor receptor signaling pathway / Assembly of collagen fibrils and other multimeric structures / endodermal cell differentiation ...
gelatinase B / negative regulation of epithelial cell differentiation involved in kidney development / negative regulation of cation channel activity / negative regulation of cysteine-type endopeptidase activity involved in apoptotic signaling pathway / cellular response to UV-A / positive regulation of keratinocyte migration / regulation of neuroinflammatory response / positive regulation of epidermal growth factor receptor signaling pathway / Assembly of collagen fibrils and other multimeric structures / endodermal cell differentiation ... gelatinase B / negative regulation of epithelial cell differentiation involved in kidney development / negative regulation of cation channel activity / negative regulation of cysteine-type endopeptidase activity involved in apoptotic signaling pathway / cellular response to UV-A / positive regulation of keratinocyte migration / regulation of neuroinflammatory response / positive regulation of epidermal growth factor receptor signaling pathway / Assembly of collagen fibrils and other multimeric structures / endodermal cell differentiation / Activation of Matrix Metalloproteinases / positive regulation of release of cytochrome c from mitochondria / response to amyloid-beta / macrophage differentiation / Collagen degradation / collagen catabolic process / EPH-ephrin mediated repulsion of cells / extracellular matrix disassembly / ephrin receptor signaling pathway / positive regulation of DNA binding / negative regulation of intrinsic apoptotic signaling pathway / positive regulation of vascular associated smooth muscle cell proliferation / cellular response to cadmium ion /
gelatinase B / negative regulation of epithelial cell differentiation involved in kidney development / negative regulation of cation channel activity / negative regulation of cysteine-type endopeptidase activity involved in apoptotic signaling pathway / cellular response to UV-A / positive regulation of keratinocyte migration / regulation of neuroinflammatory response / positive regulation of epidermal growth factor receptor signaling pathway / Assembly of collagen fibrils and other multimeric structures / endodermal cell differentiation / Activation of Matrix Metalloproteinases / positive regulation of release of cytochrome c from mitochondria / response to amyloid-beta / macrophage differentiation / Collagen degradation / collagen catabolic process / EPH-ephrin mediated repulsion of cells / extracellular matrix disassembly / ephrin receptor signaling pathway / positive regulation of DNA binding / negative regulation of intrinsic apoptotic signaling pathway / positive regulation of vascular associated smooth muscle cell proliferation / cellular response to cadmium ion /  collagen binding /
collagen binding /  embryo implantation / Degradation of the extracellular matrix / extracellular matrix organization / positive regulation of receptor binding /
embryo implantation / Degradation of the extracellular matrix / extracellular matrix organization / positive regulation of receptor binding /  skeletal system development / Signaling by SCF-KIT /
skeletal system development / Signaling by SCF-KIT /  metalloendopeptidase activity / cellular response to reactive oxygen species /
metalloendopeptidase activity / cellular response to reactive oxygen species /  metallopeptidase activity /
metallopeptidase activity /  cell migration / tertiary granule lumen /
cell migration / tertiary granule lumen /  peptidase activity / Interleukin-4 and Interleukin-13 signaling / collagen-containing extracellular matrix /
peptidase activity / Interleukin-4 and Interleukin-13 signaling / collagen-containing extracellular matrix /  endopeptidase activity / cellular response to lipopolysaccharide / ficolin-1-rich granule lumen / Extra-nuclear estrogen signaling / positive regulation of protein phosphorylation / positive regulation of apoptotic process / serine-type endopeptidase activity / apoptotic process / Neutrophil degranulation / negative regulation of apoptotic process /
endopeptidase activity / cellular response to lipopolysaccharide / ficolin-1-rich granule lumen / Extra-nuclear estrogen signaling / positive regulation of protein phosphorylation / positive regulation of apoptotic process / serine-type endopeptidase activity / apoptotic process / Neutrophil degranulation / negative regulation of apoptotic process /  proteolysis /
proteolysis /  extracellular space / extracellular exosome / zinc ion binding / extracellular region / identical protein binding
extracellular space / extracellular exosome / zinc ion binding / extracellular region / identical protein binding
 Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2 Å
MOLECULAR REPLACEMENT / Resolution: 2 Å  Authors
Authors Citation
Citation Journal: J.Mol.Biol. / Year: 2007
Journal: J.Mol.Biol. / Year: 2007 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 2ovz.cif.gz
2ovz.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb2ovz.ent.gz
pdb2ovz.ent.gz PDB format
PDB format 2ovz.json.gz
2ovz.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/ov/2ovz
https://data.pdbj.org/pub/pdb/validation_reports/ov/2ovz ftp://data.pdbj.org/pub/pdb/validation_reports/ov/2ovz
ftp://data.pdbj.org/pub/pdb/validation_reports/ov/2ovz




 Links
Links Assembly
Assembly

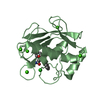

 Components
Components
 Homo sapiens (human) / Gene: MMP9, CLG4B / Production host:
Homo sapiens (human) / Gene: MMP9, CLG4B / Production host: 
 Escherichia coli (E. coli) / References: UniProt: P14780,
Escherichia coli (E. coli) / References: UniProt: P14780,  gelatinase B
gelatinase B








 Chloride
Chloride Water
Water X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation
 SYNCHROTRON / Site: MPG/DESY, HAMBURG
SYNCHROTRON / Site: MPG/DESY, HAMBURG  / Beamline: BW6 / Wavelength: 1.05
/ Beamline: BW6 / Wavelength: 1.05  : 1.05 Å / Relative weight: 1
: 1.05 Å / Relative weight: 1  Processing
Processing :
:  MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller














 PDBj
PDBj














