+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2f43 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Rat liver F1-ATPase | ||||||
 Components Components | (ATP synthase ... ) x 3 ) x 3 | ||||||
 Keywords Keywords |  HYDROLASE / HYDROLASE /  ATP SYNTHASE / F0F1-ATPASE / ATP SYNTHASE / F0F1-ATPASE /  OXIDATIVE PHOSPHORYLATION / OXIDATIVE PHOSPHORYLATION /  MITOCHONDRIA / MITOCHONDRIA /  VANADATE VANADATE | ||||||
| Function / homology |  Function and homology information Function and homology informationlipoprotein particle receptor activity /  Mitochondrial protein import / Formation of ATP by chemiosmotic coupling / Cristae formation / Mitochondrial protein import / Formation of ATP by chemiosmotic coupling / Cristae formation /  Mitochondrial protein degradation / negative regulation of cell adhesion involved in substrate-bound cell migration / response to curcumin / response to 3,3',5-triiodo-L-thyronine / cold acclimation / cellular response to peptide ...lipoprotein particle receptor activity / Mitochondrial protein degradation / negative regulation of cell adhesion involved in substrate-bound cell migration / response to curcumin / response to 3,3',5-triiodo-L-thyronine / cold acclimation / cellular response to peptide ...lipoprotein particle receptor activity /  Mitochondrial protein import / Formation of ATP by chemiosmotic coupling / Cristae formation / Mitochondrial protein import / Formation of ATP by chemiosmotic coupling / Cristae formation /  Mitochondrial protein degradation / negative regulation of cell adhesion involved in substrate-bound cell migration / response to curcumin / response to 3,3',5-triiodo-L-thyronine / cold acclimation / cellular response to peptide / ATP biosynthetic process / Mitochondrial protein degradation / negative regulation of cell adhesion involved in substrate-bound cell migration / response to curcumin / response to 3,3',5-triiodo-L-thyronine / cold acclimation / cellular response to peptide / ATP biosynthetic process /  angiostatin binding / angiostatin binding /  COP9 signalosome / proton-transporting ATP synthase complex / cellular response to interleukin-7 / response to muscle activity / response to manganese ion / mitochondrial proton-transporting ATP synthase, catalytic core / mitochondrial proton-transporting ATP synthase complex, catalytic sector F(1) / mitochondrial nucleoid / mitochondrial proton-transporting ATP synthase complex / negative regulation of endothelial cell proliferation / MHC class I protein binding / proton motive force-driven ATP synthesis / proton motive force-driven mitochondrial ATP synthesis / cellular response to nitric oxide / positive regulation of blood vessel endothelial cell migration / proton-transporting ATP synthase complex, catalytic core F(1) / COP9 signalosome / proton-transporting ATP synthase complex / cellular response to interleukin-7 / response to muscle activity / response to manganese ion / mitochondrial proton-transporting ATP synthase, catalytic core / mitochondrial proton-transporting ATP synthase complex, catalytic sector F(1) / mitochondrial nucleoid / mitochondrial proton-transporting ATP synthase complex / negative regulation of endothelial cell proliferation / MHC class I protein binding / proton motive force-driven ATP synthesis / proton motive force-driven mitochondrial ATP synthesis / cellular response to nitric oxide / positive regulation of blood vessel endothelial cell migration / proton-transporting ATP synthase complex, catalytic core F(1) /  H+-transporting two-sector ATPase / proton transmembrane transport / proton-transporting ATPase activity, rotational mechanism / proton-transporting ATP synthase activity, rotational mechanism / H+-transporting two-sector ATPase / proton transmembrane transport / proton-transporting ATPase activity, rotational mechanism / proton-transporting ATP synthase activity, rotational mechanism /  receptor-mediated endocytosis / cellular response to dexamethasone stimulus / liver development / receptor-mediated endocytosis / cellular response to dexamethasone stimulus / liver development /  mitochondrial membrane / mitochondrial membrane /  regulation of intracellular pH / regulation of intracellular pH /  ADP binding / lipid metabolic process / response to ethanol / ADP binding / lipid metabolic process / response to ethanol /  angiogenesis / angiogenesis /  protease binding / protease binding /  mitochondrial inner membrane / mitochondrial inner membrane /  membrane raft / membrane raft /  calcium ion binding / calcium ion binding /  cell surface / cell surface /  ATP hydrolysis activity / ATP hydrolysis activity /  mitochondrion / mitochondrion /  ATP binding / ATP binding /  membrane / membrane /  plasma membrane plasma membraneSimilarity search - Function | ||||||
| Biological species |   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3 Å MOLECULAR REPLACEMENT / Resolution: 3 Å | ||||||
 Authors Authors | Chen, C. / Saxena, A.K. / Simcoke, W.N. / Garboczi, D.N. / Pedersen, P.L. / Ko, Y.H. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2006 Journal: J.Biol.Chem. / Year: 2006Title: Mitochondrial ATP synthase: Crystal structure of the catalytic F1 unit in a vanadate-induced transition-like state and implications for mechanism. Authors: Chen, C. / Saxena, A.K. / Simcoke, W.N. / Garboczi, D.N. / Pedersen, P.L. / Ko, Y.H. #1:  Journal: Proc.Natl.Acad.Sci.Usa / Year: 1998 Journal: Proc.Natl.Acad.Sci.Usa / Year: 1998Title: The 2.8-A structure of rat liver F1-ATPase: configuration of a critical intermediate in ATP synthesis/hydrolysis. Authors: Bianchet, M.A. / Hullihen, J. / Pedersen, P.L. / Amzel, L.M. #2: Journal: Biochim.Biophys.Acta / Year: 1994 Title: The three-dimensional structure of rat liver mitochodria F1-ATPase: X-ray diffraction studies. Authors: Bianchet, M. / Medjahed, D. / Hulihen, J. / Pedersen, P.L. / Amzel, L.M. #3: Journal: J.Bioenerg.Biomembr. / Year: 1992 Title: Quaternary structure of ATP synthases: symmetry and asymmetry in the F1 moiety. Authors: Amzel, L.M. / Bianchet, M.A. / Pedersen, P.L. #4: Journal: J.Biol.Chem. / Year: 1991 Title: Mitochondrial ATP synthase. Quaternary structure of the F1 moiety at 3.6 A determined by x-ray diffraction analysis. Authors: Bianchet, M. / Ysern, X. / Hullihen, J. / Pedersen, P.L. / Amzel, L.M. | ||||||
| History |
| ||||||
| Remark 300 | BIOMOLECULE: 1 THIS ENTRY CONTAINS THE CRYSTALLOGRAPHIC ASYMMETRIC UNIT WHICH CONSISTS OF 3 ... BIOMOLECULE: 1 THIS ENTRY CONTAINS THE CRYSTALLOGRAPHIC ASYMMETRIC UNIT WHICH CONSISTS OF 3 CHAIN(S). SEE REMARK 350 FOR INFORMATION ON GENERATING THE BIOLOGICAL MOLECULE(S). THIS PROTEIN HAS AN UNUSUAL STOICHIOMETRY AND THUS, UNUSUAL SYMMETRY. THE BIOLOGICAL UNIT (ACTIVE ENZYME) IS COMPOSED OF 3 A CHAINS, 3 B CHAINS AND 1 G CHAIN. IN THE R32 SPACEGROUP OF THIS CRYSTAL, THE ASYMMETRIC UNIT IS MADE UP OF ONE A CHAIN, ONE B CHAIN AND ONE-THIRD OF A G CHAIN. THE G CHAIN LIES ON THE 3-FOLD CRYSTALLOGRAPHIC SYMMETRY AXIS AND EACH ASYMMETRIC UNIT CONTAINS A RANDOM ONE OF THE THREE CRYSTALLOGRAPHICALLY SYMMETRIC POSITIONS. TO MAKE A BIOMOLECULE, THE 3-FOLD MATRICES IN REMARK 350 ARE ONLY APPLIED TO CHAIN A AND CHAIN B. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2f43.cif.gz 2f43.cif.gz | 221.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2f43.ent.gz pdb2f43.ent.gz | 172.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2f43.json.gz 2f43.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/f4/2f43 https://data.pdbj.org/pub/pdb/validation_reports/f4/2f43 ftp://data.pdbj.org/pub/pdb/validation_reports/f4/2f43 ftp://data.pdbj.org/pub/pdb/validation_reports/f4/2f43 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1mabS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
|
- Components
Components
-ATP synthase ... , 3 types, 3 molecules ABG
| #1: Protein | Mass: 55334.195 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Rattus norvegicus (Norway rat) / Organ: LIVER Rattus norvegicus (Norway rat) / Organ: LIVER References: UniProt: P15999,  H+-transporting two-sector ATPase H+-transporting two-sector ATPase |
|---|---|
| #2: Protein | Mass: 51404.461 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Rattus norvegicus (Norway rat) / Organ: LIVER Rattus norvegicus (Norway rat) / Organ: LIVER References: UniProt: P10719,  H+-transporting two-sector ATPase H+-transporting two-sector ATPase |
| #3: Protein | Mass: 30231.725 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Rattus norvegicus (Norway rat) / Organ: LIVER Rattus norvegicus (Norway rat) / Organ: LIVER References: UniProt: P35435,  H+-transporting two-sector ATPase H+-transporting two-sector ATPase |
-Non-polymers , 4 types, 5 molecules 
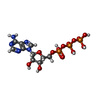





| #4: Chemical | | #5: Chemical | ChemComp-ATP / |  Adenosine triphosphate Adenosine triphosphate#6: Chemical | ChemComp-VO4 / |  Vanadate Vanadate#7: Chemical | ChemComp-ADP / |  Adenosine diphosphate Adenosine diphosphate |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.1 Å3/Da / Density % sol: 60 % |
|---|---|
Crystal grow | Temperature: 296 K / pH: 8.08 Details: 5MM ATP, 5MM MGCL2, 5MM VANADATE, 25MM NAN3, 50MM MOPS, pH 8.08, VAPOR DIFFUSION, SITTING DROP, temperature 296K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 19-ID / Wavelength: 0.99092 / Beamline: 19-ID / Wavelength: 0.99092 |
| Detector | Type: SBC-2 / Detector: CCD / Details: MONOCROMATOR |
| Radiation | Monochromator: GRAPHITE / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.99092 Å / Relative weight: 1 : 0.99092 Å / Relative weight: 1 |
| Reflection | Resolution: 3→73.52 Å / Num. obs: 29647 / % possible obs: 100 % / Redundancy: 8.7 % / Rsym value: 0.143 / Net I/σ(I): 12.1 |
| Reflection shell | Resolution: 3→3.16 Å / Redundancy: 8.9 % / Mean I/σ(I) obs: 3.2 / Rsym value: 0.75 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1MAB Resolution: 3→19.97 Å / Rfactor Rfree error: 0.008 / Data cutoff high absF: 7286337.69 / Data cutoff low absF: 0 / Isotropic thermal model: OVERALL / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: Engh & Huber
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 66.4837 Å2 / ksol: 0.277358 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 67.9 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3→19.97 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 3→3.11 Å / Total num. of bins used: 10
|
 Movie
Movie Controller
Controller













 PDBj
PDBj






