[English] 日本語
 Yorodumi
Yorodumi- PDB-2ab2: Mineralocorticoid Receptor Double Mutant with Bound Spironolactone -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2ab2 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Mineralocorticoid Receptor Double Mutant with Bound Spironolactone | ||||||
 Components Components | Mineralocorticoid receptor | ||||||
 Keywords Keywords |  TRANSCRIPTION / TRANSCRIPTION /  Mineralocorticoid receptor / MR / Mineralocorticoid receptor / MR /  Nuclear Receptor / Nuclear Receptor /  Steroid Receptor / Steroid Receptor /  Spironolactone / Spironolactone /  Hypertension Hypertension | ||||||
| Function / homology |  Function and homology information Function and homology informationnuclear steroid receptor activity /  estrogen response element binding / intracellular steroid hormone receptor signaling pathway / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / TBP-class protein binding / estrogen response element binding / intracellular steroid hormone receptor signaling pathway / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / TBP-class protein binding /  steroid binding / SUMOylation of intracellular receptors / Nuclear Receptor transcription pathway / positive regulation of non-canonical NF-kappaB signal transduction / steroid binding / SUMOylation of intracellular receptors / Nuclear Receptor transcription pathway / positive regulation of non-canonical NF-kappaB signal transduction /  nuclear receptor activity ...nuclear steroid receptor activity / nuclear receptor activity ...nuclear steroid receptor activity /  estrogen response element binding / intracellular steroid hormone receptor signaling pathway / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / TBP-class protein binding / estrogen response element binding / intracellular steroid hormone receptor signaling pathway / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / TBP-class protein binding /  steroid binding / SUMOylation of intracellular receptors / Nuclear Receptor transcription pathway / positive regulation of non-canonical NF-kappaB signal transduction / steroid binding / SUMOylation of intracellular receptors / Nuclear Receptor transcription pathway / positive regulation of non-canonical NF-kappaB signal transduction /  nuclear receptor activity / sequence-specific double-stranded DNA binding / nuclear receptor activity / sequence-specific double-stranded DNA binding /  receptor complex / DNA-binding transcription factor activity, RNA polymerase II-specific / DNA-binding transcription factor activity / receptor complex / DNA-binding transcription factor activity, RNA polymerase II-specific / DNA-binding transcription factor activity /  chromatin / endoplasmic reticulum membrane / regulation of transcription by RNA polymerase II / chromatin / endoplasmic reticulum membrane / regulation of transcription by RNA polymerase II /  signal transduction / zinc ion binding / signal transduction / zinc ion binding /  nucleoplasm / nucleoplasm /  cytosol cytosolSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.85 Å MOLECULAR REPLACEMENT / Resolution: 1.85 Å | ||||||
 Authors Authors | Bledsoe, R.K. / Madauss, K.P. / Holt, J.A. / Apolito, C.J. / Lambert, M.H. / Pearce, K.H. / Stanley, T.B. / Stewart, E.L. / Trump, R.P. / Willson, T.M. / Williams, S.P. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2005 Journal: J.Biol.Chem. / Year: 2005Title: A Ligand-mediated Hydrogen Bond Network Required for the Activation of the Mineralocorticoid Receptor Authors: Bledsoe, R.K. / Madauss, K.P. / Holt, J.A. / Apolito, C.J. / Lambert, M.H. / Pearce, K.H. / Stanley, T.B. / Stewart, E.L. / Trump, R.P. / Willson, T.M. / Williams, S.P. | ||||||
| History |
| ||||||
| Remark 300 | BIOMOLECULE: 1 THIS ENTRY CONTAINS THE CRYSTALLOGRAPHIC ASYMMETRIC UNIT WHICH CONSISTS OF 2 CHAIN(S) ...BIOMOLECULE: 1 THIS ENTRY CONTAINS THE CRYSTALLOGRAPHIC ASYMMETRIC UNIT WHICH CONSISTS OF 2 CHAIN(S). THE BIOLOGICAL UNIT IS A DIMER, BUT THE DIMER IN THE CRYSTAL IS IRRELEVANT BIOLOGICALLY. THE BIOLOGICAL DIMER IS CURRENTLY UNKNOWN. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2ab2.cif.gz 2ab2.cif.gz | 125.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2ab2.ent.gz pdb2ab2.ent.gz | 96.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2ab2.json.gz 2ab2.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ab/2ab2 https://data.pdbj.org/pub/pdb/validation_reports/ab/2ab2 ftp://data.pdbj.org/pub/pdb/validation_reports/ab/2ab2 ftp://data.pdbj.org/pub/pdb/validation_reports/ab/2ab2 | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Details | The biological unit is a dimer, but the non-crystallographic symmetry observed is biologically irrelevant |
- Components
Components
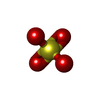
| #1: Protein |  / MR / MRMass: 31636.598 Da / Num. of mol.: 2 / Fragment: MR Ligand Binding Domain / Mutation: C808S, S810L Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: NR3C2, MCR, MLR / Plasmid: pHis GST / Species (production host): Escherichia coli / Production host: Homo sapiens (human) / Gene: NR3C2, MCR, MLR / Plasmid: pHis GST / Species (production host): Escherichia coli / Production host:   Escherichia coli BL21(DE3) (bacteria) / Strain (production host): BL21(DE3) / References: UniProt: P08235 Escherichia coli BL21(DE3) (bacteria) / Strain (production host): BL21(DE3) / References: UniProt: P08235#2: Chemical |  Spironolactone Spironolactone#3: Chemical | ChemComp-SO4 / |  Sulfate Sulfate#4: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.8 Å3/Da / Density % sol: 56 % |
|---|---|
Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 7.5 Details: 0.1m Hepes pH7.5, 0.9M Li2SO4, 2% PEG 2KMME, vapor diffusion, hanging drop, temperature 298K |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 17-ID / Wavelength: 1 Å / Beamline: 17-ID / Wavelength: 1 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: ADSC QUANTUM 210 / Detector: CCD / Date: Jul 3, 2003 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Monochromator: SAGITALLY FOCUSED Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength : 1 Å / Relative weight: 1 : 1 Å / Relative weight: 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Redundancy: 4.6 % / Number: 54805 / Rmerge(I) obs: 0.063 / Χ2: 1.08 / D res high: 1.85 Å / D res low: 50 Å / % possible obs: 96.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Diffraction reflection shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.85→20 Å / Num. all: 56823 / Num. obs: 54805 / % possible obs: 96.5 % / Observed criterion σ(F): 1 / Observed criterion σ(I): 1 / Redundancy: 4.6 % / Rmerge(I) obs: 0.063 / Χ2: 1.08 / Net I/σ(I): 22.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Resolution: 1.85→1.92 Å / % possible obs: 83.7 % / Redundancy: 2.6 % / Rmerge(I) obs: 0.395 / Mean I/σ(I) obs: 2.6 / Num. measured obs: 4694 / Num. unique all: 4694 / Χ2: 1.192 / % possible all: 83.7 |
-Phasing
| Phasing MR | Rfactor: 57.2 / Cor.coef. Fo:Fc: 21.8 / Cor.coef. Io to Ic: 27.1
|
|---|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: MR LBD Resolution: 1.85→20 Å / Isotropic thermal model: Isotropic / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: Engh & Huber
| ||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 20.024 Å2
| ||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.85→20 Å
| ||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||
| Xplor file |
|
 Movie
Movie Controller
Controller

















 PDBj
PDBj