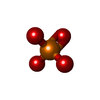[English] 日本語
 Yorodumi
Yorodumi- PDB-1rv3: E75L MUTANT OF RABBIT CYTOSOLIC SERINE HYDROXYMETHYLTRANSFERASE, ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1rv3 | ||||||
|---|---|---|---|---|---|---|---|
| Title | E75L MUTANT OF RABBIT CYTOSOLIC SERINE HYDROXYMETHYLTRANSFERASE, COMPLEX WITH GLYCINE | ||||||
 Components Components | Serine hydroxymethyltransferase, cytosolic | ||||||
 Keywords Keywords |  TRANSFERASE / ONE-CARBON METABOLISM TRANSFERASE / ONE-CARBON METABOLISM | ||||||
| Function / homology |  Function and homology information Function and homology informationcellular response to tetrahydrofolate / purine nucleobase biosynthetic process /  serine binding / L-serine metabolic process / L-serine catabolic process / glycine metabolic process / serine binding / L-serine metabolic process / L-serine catabolic process / glycine metabolic process /  glycine hydroxymethyltransferase / glycine hydroxymethyltransferase /  glycine hydroxymethyltransferase activity / glycine biosynthetic process from serine / tetrahydrofolate metabolic process ...cellular response to tetrahydrofolate / purine nucleobase biosynthetic process / glycine hydroxymethyltransferase activity / glycine biosynthetic process from serine / tetrahydrofolate metabolic process ...cellular response to tetrahydrofolate / purine nucleobase biosynthetic process /  serine binding / L-serine metabolic process / L-serine catabolic process / glycine metabolic process / serine binding / L-serine metabolic process / L-serine catabolic process / glycine metabolic process /  glycine hydroxymethyltransferase / glycine hydroxymethyltransferase /  glycine hydroxymethyltransferase activity / glycine biosynthetic process from serine / tetrahydrofolate metabolic process / tetrahydrofolate interconversion / folic acid metabolic process / mRNA regulatory element binding translation repressor activity / mRNA 5'-UTR binding / glycine hydroxymethyltransferase activity / glycine biosynthetic process from serine / tetrahydrofolate metabolic process / tetrahydrofolate interconversion / folic acid metabolic process / mRNA regulatory element binding translation repressor activity / mRNA 5'-UTR binding /  pyridoxal phosphate binding / protein homotetramerization / protein homodimerization activity / pyridoxal phosphate binding / protein homotetramerization / protein homodimerization activity /  nucleoplasm / nucleoplasm /  cytosol cytosolSimilarity search - Function | ||||||
| Biological species |   Oryctolagus cuniculus (rabbit) Oryctolagus cuniculus (rabbit) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.4 Å MOLECULAR REPLACEMENT / Resolution: 2.4 Å | ||||||
 Authors Authors | Szebenyi, D.M. / Musayev, F.N. / di Salvo, M.L. / Safo, M.K. / Schirch, V. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2004 Journal: Biochemistry / Year: 2004Title: Serine Hydroxymethyltransferase: Role of Glu75 and Evidence that Serine Is Cleaved by a Retroaldol Mechanism. Authors: Szebenyi, D.M. / Musayev, F.N. / di Salvo, M.L. / Safo, M.K. / Schirch, V. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1rv3.cif.gz 1rv3.cif.gz | 189.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1rv3.ent.gz pdb1rv3.ent.gz | 150.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1rv3.json.gz 1rv3.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/rv/1rv3 https://data.pdbj.org/pub/pdb/validation_reports/rv/1rv3 ftp://data.pdbj.org/pub/pdb/validation_reports/rv/1rv3 ftp://data.pdbj.org/pub/pdb/validation_reports/rv/1rv3 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1rv4C  1rvuC  1rvyC  1cj0S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
| ||||||||
| Details | The asymmetric unit contains a homodimer. The biological assembly is a tetramer consisting of a pair of dimers. The second dimer is generated by the operation: y,x,1-z. |
- Components
Components
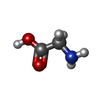
| #1: Protein |  / Serine methylase / Glycine hydroxymethyltransferase / SHMT / Serine methylase / Glycine hydroxymethyltransferase / SHMTMass: 52900.156 Da / Num. of mol.: 2 / Mutation: E75L Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Oryctolagus cuniculus (rabbit) / Gene: SHMT1 / Plasmid: pET22b / Production host: Oryctolagus cuniculus (rabbit) / Gene: SHMT1 / Plasmid: pET22b / Production host:   Escherichia coli (E. coli) Escherichia coli (E. coli)References: UniProt: P07511,  glycine hydroxymethyltransferase glycine hydroxymethyltransferase#2: Chemical |  Phosphate Phosphate#3: Chemical |  Pyridoxal phosphate Pyridoxal phosphate#4: Chemical | ChemComp-GLY / |  Glycine Glycine#5: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.58 Å3/Da / Density % sol: 51.91 % |
|---|---|
Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 6.6 Details: PEG 8000, KCl, potassium phosphate, pH 6.6, VAPOR DIFFUSION, HANGING DROP, temperature 298.0K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  CHESS CHESS  / Beamline: A1 / Wavelength: 0.9363 Å / Beamline: A1 / Wavelength: 0.9363 Å |
| Detector | Type: ADSC QUANTUM 210 / Detector: CCD / Date: Feb 6, 2003 / Details: White beam mirror, focussing monochromatic mirror |
| Radiation | Monochromator: SI 111, bent triangle / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.9363 Å / Relative weight: 1 : 0.9363 Å / Relative weight: 1 |
| Reflection | Resolution: 2.4→48.9 Å / Num. all: 42150 / Num. obs: 42006 / % possible obs: 99.8 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 13 % / Biso Wilson estimate: 51.8 Å2 / Rsym value: 0.1 / Net I/σ(I): 6 |
| Reflection shell | Resolution: 2.4→2.53 Å / Redundancy: 12.2 % / Mean I/σ(I) obs: 1.3 / Num. unique all: 5998 / Rsym value: 0.566 / % possible all: 99.7 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1CJ0 Resolution: 2.4→48.88 Å / Rfactor Rfree error: 0.006 / Data cutoff high absF: 2595470.56 / Data cutoff low absF: 0 / Isotropic thermal model: GROUP / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: Engh & Huber
| |||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 31.7549 Å2 / ksol: 0.313623 e/Å3 | |||||||||||||||||||||||||
| Displacement parameters | Biso mean: 48.6 Å2
| |||||||||||||||||||||||||
| Refine analyze |
| |||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.4→48.88 Å
| |||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.4→2.55 Å / Rfactor Rfree error: 0.019 / Total num. of bins used: 6
| |||||||||||||||||||||||||
| Xplor file |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj