+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
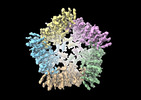
| Title | Human TRPV3 tetramer structure, closed conformation | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  Ion Channel / Ion Channel /  TRP Channel / Closed Conformation / TRP Channel / Closed Conformation /  MEMBRANE PROTEIN MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of hair cycle /  TRP channels / response to temperature stimulus / positive regulation of calcium ion import / calcium ion transmembrane transport / TRP channels / response to temperature stimulus / positive regulation of calcium ion import / calcium ion transmembrane transport /  calcium channel activity / calcium channel activity /  receptor complex / identical protein binding / receptor complex / identical protein binding /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
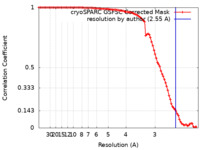
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.55 Å cryo EM / Resolution: 2.55 Å | |||||||||
 Authors Authors | Lansky S / Betancourt JM / Scheuring S | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: A pentameric TRPV3 channel with a dilated pore. Authors: Shifra Lansky / John Michael Betancourt / Jingying Zhang / Yining Jiang / Elizabeth D Kim / Navid Paknejad / Crina M Nimigean / Peng Yuan / Simon Scheuring /  Abstract: Transient receptor potential (TRP) channels are a large, eukaryotic ion channel superfamily that control diverse physiological functions, and therefore are attractive drug targets. More than 210 ...Transient receptor potential (TRP) channels are a large, eukaryotic ion channel superfamily that control diverse physiological functions, and therefore are attractive drug targets. More than 210 structures from more than 20 different TRP channels have been determined, and all are tetramers. Despite this wealth of structures, many aspects concerning TRPV channels remain poorly understood, including the pore-dilation phenomenon, whereby prolonged activation leads to increased conductance, permeability to large ions and loss of rectification. Here, we used high-speed atomic force microscopy (HS-AFM) to analyse membrane-embedded TRPV3 at the single-molecule level and discovered a pentameric state. HS-AFM dynamic imaging revealed transience and reversibility of the pentamer in dynamic equilibrium with the canonical tetramer through membrane diffusive protomer exchange. The pentamer population increased upon diphenylboronic anhydride (DPBA) addition, an agonist that has been shown to induce TRPV3 pore dilation. On the basis of these findings, we designed a protein production and data analysis pipeline that resulted in a cryogenic-electron microscopy structure of the TRPV3 pentamer, showing an enlarged pore compared to the tetramer. The slow kinetics to enter and exit the pentameric state, the increased pentamer formation upon DPBA addition and the enlarged pore indicate that the pentamer represents the structural correlate of pore dilation. We thus show membrane diffusive protomer exchange as an additional mechanism for structural changes and conformational variability. Overall, we provide structural evidence for a non-canonical pentameric TRP-channel assembly, laying the foundation for new directions in TRP channel research. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40181.map.gz emd_40181.map.gz | 79 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40181-v30.xml emd-40181-v30.xml emd-40181.xml emd-40181.xml | 18.3 KB 18.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_40181_fsc.xml emd_40181_fsc.xml | 9.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_40181.png emd_40181.png | 77 KB | ||
| Masks |  emd_40181_msk_1.map emd_40181_msk_1.map | 83.7 MB |  Mask map Mask map | |
| Others |  emd_40181_half_map_1.map.gz emd_40181_half_map_1.map.gz emd_40181_half_map_2.map.gz emd_40181_half_map_2.map.gz | 77.3 MB 77.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40181 http://ftp.pdbj.org/pub/emdb/structures/EMD-40181 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40181 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40181 | HTTPS FTP |
-Related structure data
| Related structure data |  8gkaMC  8gkgC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_40181.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40181.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.076 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_40181_msk_1.map emd_40181_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_40181_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_40181_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : TRPV3 ion channel- tetrameric oligomerization assembly
| Entire | Name: TRPV3 ion channel- tetrameric oligomerization assembly |
|---|---|
| Components |
|
-Supramolecule #1: TRPV3 ion channel- tetrameric oligomerization assembly
| Supramolecule | Name: TRPV3 ion channel- tetrameric oligomerization assembly type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Transient receptor potential cation channel subfamily V member 3
| Macromolecule | Name: Transient receptor potential cation channel subfamily V member 3 type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 90.742812 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MKAHPKEMVP LMGKRVAAPS GNPAILPEKR PAEITPTKKS AHFFLEIEGF EPNPTVAKTS PPVFSKPMDS NIRQCISGNC DDMDSPQSP QDDVTETPSN PNSPSAQLAK EEQRRKKRRL KKRIFAAVSE GCVEELVELL VELQELCRRR HDEDVPDFLM H KLTASDTG ...String: MKAHPKEMVP LMGKRVAAPS GNPAILPEKR PAEITPTKKS AHFFLEIEGF EPNPTVAKTS PPVFSKPMDS NIRQCISGNC DDMDSPQSP QDDVTETPSN PNSPSAQLAK EEQRRKKRRL KKRIFAAVSE GCVEELVELL VELQELCRRR HDEDVPDFLM H KLTASDTG KTCLMKALLN INPNTKEIVR ILLAFAEEND ILGRFINAEY TEEAYEGQTA LNIAIERRQG DIAALLIAAG AD VNAHAKG AFFNPKYQHE GFYFGETPLA LAACTNQPEI VQLLMEHEQT DITSRDSRGN NILHALVTVA EDFKTQNDFV KRM YDMILL RSGNWELETT RNNDGLTPLQ LAAKMGKAEI LKYILSREIK EKRLRSLSRK FTDWAYGPVS SSLYDLTNVD TTTD NSVLE ITVYNTNIDN RHEMLTLEPL HTLLHMKWKK FAKHMFFLSF CFYFFYNITL TLVSYYRPRE EEAIPHPLAL THKMG WLQL LGRMFVLIWA MCISVKEGIA IFLLRPSDLQ SILSDAWFHF VFFIQAVLVI LSVFLYLFAY KEYLACLVLA MALGWA NML YYTRGFQSMG MYSVMIQKVI LHDVLKFLFV YIVFLLGFGV ALASLIEKCP KDNKDCSSYG SFSDAVLELF KLTIGLG DL NIQQNSKYPI LFLFLLITYV ILTFVLLLNM LIALMGETVE NVSKESERIW RLQRARTILE FEKMLPEWLR SRFRMGEL C KVAEDDFRLC LRINEVKWTE WKTHVSFLNE DPGPVRRTDF NKIQDSSRNN SKTTLNAFEE VEEFPETSV UniProtKB: Transient receptor potential cation channel subfamily V member 3 |
-Macromolecule #2: SODIUM ION
| Macromolecule | Name: SODIUM ION / type: ligand / ID: 2 / Number of copies: 1 |
|---|---|
| Molecular weight | Theoretical: 22.99 Da |
-Macromolecule #3: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(tri...
| Macromolecule | Name: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(trimethylammonio)ethyl phosphate type: ligand / ID: 3 / Number of copies: 33 / Formula: POV |
|---|---|
| Molecular weight | Theoretical: 760.076 Da |
| Chemical component information | 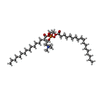 ChemComp-POV: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
Details: 20 mM Tris pH 8, 150 mM NaCl, 0.01% GDN, 1 mM beta-mercaptoethanol | |||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 400 / Pretreatment - Type: GLOW DISCHARGE | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: 3 ul sample blot 2 sec force 3 wait 20 sec. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 0.001 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 64000 Bright-field microscopy / Cs: 0.001 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 64000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 2 / Number real images: 10125 / Average electron dose: 53.27 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z
Z Y
Y X
X