+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3773 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | High resolution INO80 core complex | |||||||||
 Map data Map data | High resolution human INO80 core complex. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  Chromatin remodelling complex / Chromatin remodelling complex /  GENE REGULATION GENE REGULATION | |||||||||
| Function / homology |  Function and homology information Function and homology informationpromoter-enhancer loop anchoring activity / regulation of DNA strand elongation / positive regulation of telomere maintenance in response to DNA damage / establishment of protein localization to chromatin / R2TP complex / Swr1 complex / dynein axonemal particle / RPAP3/R2TP/prefoldin-like complex / regulation of double-strand break repair / positive regulation of telomerase RNA localization to Cajal body ...promoter-enhancer loop anchoring activity / regulation of DNA strand elongation / positive regulation of telomere maintenance in response to DNA damage / establishment of protein localization to chromatin / R2TP complex / Swr1 complex / dynein axonemal particle / RPAP3/R2TP/prefoldin-like complex / regulation of double-strand break repair / positive regulation of telomerase RNA localization to Cajal body / Ino80 complex / box C/D snoRNP assembly / protein folding chaperone complex /  NuA4 histone acetyltransferase complex / regulation of chromosome organization / positive regulation of double-strand break repair via homologous recombination / NuA4 histone acetyltransferase complex / regulation of chromosome organization / positive regulation of double-strand break repair via homologous recombination /  regulation of DNA replication / regulation of DNA replication /  MLL1 complex / TFIID-class transcription factor complex binding / MLL1 complex / TFIID-class transcription factor complex binding /  regulation of embryonic development / Telomere Extension By Telomerase / RNA polymerase II core promoter sequence-specific DNA binding / regulation of embryonic development / Telomere Extension By Telomerase / RNA polymerase II core promoter sequence-specific DNA binding /  regulation of DNA repair / Deposition of new CENPA-containing nucleosomes at the centromere / positive regulation of DNA repair / TBP-class protein binding / regulation of DNA repair / Deposition of new CENPA-containing nucleosomes at the centromere / positive regulation of DNA repair / TBP-class protein binding /  DNA helicase activity / DNA helicase activity /  telomere maintenance / cellular response to estradiol stimulus / telomere maintenance / cellular response to estradiol stimulus /  ADP binding / Formation of the beta-catenin:TCF transactivating complex / DNA Damage Recognition in GG-NER / ADP binding / Formation of the beta-catenin:TCF transactivating complex / DNA Damage Recognition in GG-NER /  euchromatin / negative regulation of canonical Wnt signaling pathway / chromatin DNA binding / euchromatin / negative regulation of canonical Wnt signaling pathway / chromatin DNA binding /  beta-catenin binding / beta-catenin binding /  nuclear matrix / positive regulation of canonical Wnt signaling pathway / transcription corepressor activity / UCH proteinases / cellular response to UV / unfolded protein binding / nuclear matrix / positive regulation of canonical Wnt signaling pathway / transcription corepressor activity / UCH proteinases / cellular response to UV / unfolded protein binding /  nucleosome / nucleosome /  protein folding / HATs acetylate histones / protein folding / HATs acetylate histones /  ATPase binding / ATPase binding /  spermatogenesis / regulation of apoptotic process / DNA recombination / spermatogenesis / regulation of apoptotic process / DNA recombination /  DNA helicase / DNA helicase /  transcription coactivator activity / protein stabilization / transcription coactivator activity / protein stabilization /  regulation of cell cycle / Ub-specific processing proteases / regulation of cell cycle / Ub-specific processing proteases /  chromatin remodeling / chromatin remodeling /  cadherin binding / cadherin binding /  cell cycle / cell cycle /  ribonucleoprotein complex / RNA polymerase II cis-regulatory region sequence-specific DNA binding / ribonucleoprotein complex / RNA polymerase II cis-regulatory region sequence-specific DNA binding /  cell division / cell division /  DNA repair / DNA repair /  centrosome / regulation of DNA-templated transcription / regulation of transcription by RNA polymerase II / positive regulation of DNA-templated transcription / centrosome / regulation of DNA-templated transcription / regulation of transcription by RNA polymerase II / positive regulation of DNA-templated transcription /  ATP hydrolysis activity / protein homodimerization activity / positive regulation of transcription by RNA polymerase II / extracellular exosome / ATP hydrolysis activity / protein homodimerization activity / positive regulation of transcription by RNA polymerase II / extracellular exosome /  nucleoplasm / nucleoplasm /  ATP binding / ATP binding /  membrane / identical protein binding / membrane / identical protein binding /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 4.06 Å cryo EM / Resolution: 4.06 Å | |||||||||
 Authors Authors | Aramayo RJ / Bythell-Douglas R / Ayala R / Willhoft O / Wigley D / Zhang X | |||||||||
| Funding support |  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2018 Journal: Nat Struct Mol Biol / Year: 2018Title: Cryo-EM structures of the human INO80 chromatin-remodeling complex. Authors: Ricardo J Aramayo / Oliver Willhoft / Rafael Ayala / Rohan Bythell-Douglas / Dale B Wigley / Xiaodong Zhang /  Abstract: Access to chromatin for processes such as transcription and DNA repair requires the sliding of nucleosomes along DNA. This process is aided by chromatin-remodeling complexes, such as the multisubunit ...Access to chromatin for processes such as transcription and DNA repair requires the sliding of nucleosomes along DNA. This process is aided by chromatin-remodeling complexes, such as the multisubunit INO80 chromatin-remodeling complex. Here we present cryo-EM structures of the active core complex of human INO80 at 9.6 Å, with portions at 4.1-Å resolution, and reconstructions of combinations of subunits. Together, these structures reveal the architecture of the INO80 complex, including Ino80 and actin-related proteins, which is assembled around a single RUVBL1 (Tip49a) and RUVBL2 (Tip49b) AAA+ heterohexamer. An unusual spoked-wheel structural domain of the Ino80 subunit is engulfed by this heterohexamer; both, in combination, form the core of the complex. We also identify a cleft in RUVBL1 and RUVBL2, which forms a major interaction site for partner proteins and probably communicates these interactions to its nucleotide-binding sites. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3773.map.gz emd_3773.map.gz | 9.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3773-v30.xml emd-3773-v30.xml emd-3773.xml emd-3773.xml | 13.2 KB 13.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_3773_fsc.xml emd_3773_fsc.xml | 11.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_3773.png emd_3773.png | 51.4 KB | ||
| Filedesc metadata |  emd-3773.cif.gz emd-3773.cif.gz | 5.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3773 http://ftp.pdbj.org/pub/emdb/structures/EMD-3773 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3773 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3773 | HTTPS FTP |
-Related structure data
| Related structure data |  5oafMC  3772C  3774C  3775C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_3773.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3773.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | High resolution human INO80 core complex. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Human INO80 core complex
| Entire | Name: Human INO80 core complex |
|---|---|
| Components |
|
-Supramolecule #1: Human INO80 core complex
| Supramolecule | Name: Human INO80 core complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 800 KDa |
-Macromolecule #1: RuvB-like 1
| Macromolecule | Name: RuvB-like 1 / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO / EC number:  DNA helicase DNA helicase |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 50.296914 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MKIEEVKSTT KTQRIASHSH VKGLGLDESG LAKQAASGLV GQENAREACG VIVELIKSKK MAGRAVLLAG PPGTGKTALA LAIAQELGS KVPFCPMVGS EVYSTEIKKT EVLMENFRRA IGLRIKETKE VYEGEVTELT PCETENPMGG YGKTISHVII G LKTAKGTK ...String: MKIEEVKSTT KTQRIASHSH VKGLGLDESG LAKQAASGLV GQENAREACG VIVELIKSKK MAGRAVLLAG PPGTGKTALA LAIAQELGS KVPFCPMVGS EVYSTEIKKT EVLMENFRRA IGLRIKETKE VYEGEVTELT PCETENPMGG YGKTISHVII G LKTAKGTK QLKLDPSIFE SLQKERVEAG DVIYIEANSG AVKRQGRCDT YATEFDLEAE EYVPLPKGDV HKKKEIIQDV TL HDLDVAN ARPQGGQDIL SMMGQLMKPK KTEITDKLRG EINKVVNKYI DQGIAELVPG VLFVDEVHML DIECFTYLHR ALE SSIAPI VIFASNRGNC VIRGTEDITS PHGIPLDLLD RVMIIRTMLY TPQEMKQIIK IRAQTEGINI SEEALNHLGE IGTK TTLRY SVQLLTPANL LAKINGKDSI EKEHVEEISE LFYDAKSSAK ILADQQDKYM K UniProtKB:  RuvB-like 1 RuvB-like 1 |
-Macromolecule #2: RuvB-like 2
| Macromolecule | Name: RuvB-like 2 / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO / EC number:  DNA helicase DNA helicase |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 51.222465 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MATVTATTKV PEIRDVTRIE RIGAHSHIRG LGLDDALEPR QASQGMVGQL AARRAAGVVL EMIREGKIAG RAVLIAGQPG TGKTAIAMG MAQALGPDTP FTAIAGSEIF SLEMSKTEAL TQAFRRSIGV RIKEETEIIE GEVVEIQIDR PATGTGSKVG K LTLKTTEM ...String: MATVTATTKV PEIRDVTRIE RIGAHSHIRG LGLDDALEPR QASQGMVGQL AARRAAGVVL EMIREGKIAG RAVLIAGQPG TGKTAIAMG MAQALGPDTP FTAIAGSEIF SLEMSKTEAL TQAFRRSIGV RIKEETEIIE GEVVEIQIDR PATGTGSKVG K LTLKTTEM ETIYDLGTKM IESLTKDKVQ AGDVITIDKA TGKISKLGRS FTRARDYDAM GSQTKFVQCP DGELQKRKEV VH TVSLHEI DVINSRTQGF LALFSGDTGE IKSEVREQIN AKVAEWREEG KAEIIPGVLF IDEVHMLDIE SFSFLNRALE SDM APVLIM ATNRGITRIR GTSYQSPHGI PIDLLDRLLI VSTTPYSEKD TKQILRIRCE EEDVEMSEDA YTVLTRIGLE TSLR YAIQL ITAASLVCRK RKGTEVQVDD IKRVYSLFLD ESRSTQYMKE YQDAFLFNEL KGETMDTS UniProtKB: RuvB-like 2 |
-Macromolecule #3: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 6 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information | 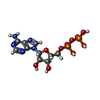 ChemComp-ADP: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Support film - Material: CARBON / Support film - topology: HOLEY |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm Bright-field microscopy / Cs: 2.7 mm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 44.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
|---|---|
| Output model |  PDB-5oaf: |
 Movie
Movie Controller
Controller














