+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the prokaryotic SPARSA system complex | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | SPARSA antiviral system / NADase /  Argonaute proteins / Argonaute proteins /  ANTIVIRAL PROTEIN ANTIVIRAL PROTEIN | ||||||||||||
| Function / homology | SIR2-like domain / SIR2-like domain / DHS-like NAD/FAD-binding domain superfamily / Ribonuclease H superfamily /  nucleic acid binding / Ribonuclease H-like superfamily / nucleic acid binding / Ribonuclease H-like superfamily /  Piwi domain protein / Sir2 superfamily protein Piwi domain protein / Sir2 superfamily protein Function and homology information Function and homology information | ||||||||||||
| Biological species |   Geobacter sulfurreducens PCA (bacteria) / Geobacter sulfurreducens PCA (bacteria) /   Geobacter sulfurreducens (bacteria) Geobacter sulfurreducens (bacteria) | ||||||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.6 Å cryo EM / Resolution: 3.6 Å | ||||||||||||
 Authors Authors | Xu X / Zhen X / Long F | ||||||||||||
| Funding support |  China, 3 items China, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structural basis of antiphage immunity generated by a prokaryotic Argonaute-associated SPARSA system. Authors: Xiangkai Zhen / Xiaolong Xu / Le Ye / Song Xie / Zhijie Huang / Sheng Yang / Yanhui Wang / Jinyu Li / Feng Long / Songying Ouyang /  Abstract: Argonaute (Ago) proteins are ubiquitous across all kingdoms of life. Eukaryotic Agos (eAgos) use small RNAs to recognize transcripts for RNA silencing in eukaryotes. In contrast, the functions of ...Argonaute (Ago) proteins are ubiquitous across all kingdoms of life. Eukaryotic Agos (eAgos) use small RNAs to recognize transcripts for RNA silencing in eukaryotes. In contrast, the functions of prokaryotic counterparts (pAgo) are less well known. Recently, short pAgos in conjunction with the associated TIR or Sir2 (SPARTA or SPARSA) were found to serve as antiviral systems to combat phage infections. Herein, we present the cryo-EM structures of nicotinamide adenine dinucleotide (NAD)-bound SPARSA with and without nucleic acids at resolutions of 3.1 Å and 3.6 Å, respectively. Our results reveal that the APAZ (Analogue of PAZ) domain and the short pAgo form a featured architecture similar to the long pAgo to accommodate nucleic acids. We further identified the key residues for NAD binding and elucidated the structural basis for guide RNA and target DNA recognition. Using structural comparisons, molecular dynamics simulations, and biochemical experiments, we proposed a putative mechanism for NAD hydrolysis in which an H186 loop mediates nucleophilic attack by catalytic water molecules. Overall, our study provides mechanistic insight into the antiphage role of the SPARSA system. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36384.map.gz emd_36384.map.gz | 6.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36384-v30.xml emd-36384-v30.xml emd-36384.xml emd-36384.xml | 18.7 KB 18.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_36384_fsc.xml emd_36384_fsc.xml | 9.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_36384.png emd_36384.png | 69.5 KB | ||
| Masks |  emd_36384_msk_1.map emd_36384_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-36384.cif.gz emd-36384.cif.gz | 6.5 KB | ||
| Others |  emd_36384_half_map_1.map.gz emd_36384_half_map_1.map.gz emd_36384_half_map_2.map.gz emd_36384_half_map_2.map.gz | 59.4 MB 59.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36384 http://ftp.pdbj.org/pub/emdb/structures/EMD-36384 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36384 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36384 | HTTPS FTP |
-Related structure data
| Related structure data |  8jkzMC  8jl0C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36384.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36384.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.84 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_36384_msk_1.map emd_36384_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_36384_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_36384_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Sir2/Ago
| Entire | Name: Sir2/Ago |
|---|---|
| Components |
|
-Supramolecule #1: Sir2/Ago
| Supramolecule | Name: Sir2/Ago / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:   Geobacter sulfurreducens PCA (bacteria) Geobacter sulfurreducens PCA (bacteria) |
-Supramolecule #2: Sir2
| Supramolecule | Name: Sir2 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Geobacter sulfurreducens PCA (bacteria) Geobacter sulfurreducens PCA (bacteria) |
-Supramolecule #3: Ago
| Supramolecule | Name: Ago / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|
-Macromolecule #1: Sir2 superfamily protein
| Macromolecule | Name: Sir2 superfamily protein / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Geobacter sulfurreducens (bacteria) Geobacter sulfurreducens (bacteria) |
| Molecular weight | Theoretical: 66.2685 KDa |
| Recombinant expression | Organism:   Escherichia coli BL21(DE3) (bacteria) Escherichia coli BL21(DE3) (bacteria) |
| Sequence | String: MDVLTDNEFY QHYLQNSQHM MWFLGAGTSR SAGLPTASDI IWDLKHRYYC LHENQDYQKH DINNHAIKSK IQSYMDSKGF PLQWSPEEY SFYFELVFRD DYEAQRKYLL EALASRKVSL NIGHRVLAAL LEMNQTKVVF TTNFDDVIET AFSDISGKHL S VYHLEGSY ...String: MDVLTDNEFY QHYLQNSQHM MWFLGAGTSR SAGLPTASDI IWDLKHRYYC LHENQDYQKH DINNHAIKSK IQSYMDSKGF PLQWSPEEY SFYFELVFRD DYEAQRKYLL EALASRKVSL NIGHRVLAAL LEMNQTKVVF TTNFDDVIET AFSDISGKHL S VYHLEGSY AALSALNTEA FPIYAKIHGD FRYQKIKNLT PDLQTNDREI HKCFLAAAIR FGLVVSGYSG RDENVMTMLR AA IDQNNAF PHGLYWTVPS ISKSEPAVQD LITYAQGKGV RAYLVETGTF DEMLSKIWRQ VKDKPAAIDA KVRTARVCPV SIP LPGPGK SFPALRTNAL PVVTQSIRCG VVTLASPITF SELKERISQK SPKALLTYTE KVLFLGGEPE IRKIFSNDEI NSIG QYYID EIAQSVAAST FLKSFVEEAI LTALLREKPI LHRVRHRTHY AVIPNASAKD DRFLDLRKAV GFKGDLGYIT GNVTN AKEL SWAEAVSIRL EERGGKLWIM LKPEIWIKPL DRREEATDFI RSRRRYRFNQ CSYQILDAWI KILFGSIGGG GTVNIS CFP DAEFKAEFEI GTRTAFSLGV G UniProtKB: Sir2 superfamily protein |
-Macromolecule #2: Piwi domain protein
| Macromolecule | Name: Piwi domain protein / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Geobacter sulfurreducens (bacteria) Geobacter sulfurreducens (bacteria) |
| Molecular weight | Theoretical: 53.325566 KDa |
| Recombinant expression | Organism:   Escherichia coli BL21(DE3) (bacteria) Escherichia coli BL21(DE3) (bacteria) |
| Sequence | String: MADNLSQLAA HSTIPEPLLL FKDNRTDTHP LRGLSQYGPY SACFNLPGQV RLAYLAPTEH MRKLDAIVRE LQNPATPKEA TNYYVEYGG FEKVFKVPLV MPQEHLRCLA LDECHGVAAN GNGLALADKI VQSMSGLFRQ KHAFDVLLVY LPASWKKCFE Y DGFDLHDR ...String: MADNLSQLAA HSTIPEPLLL FKDNRTDTHP LRGLSQYGPY SACFNLPGQV RLAYLAPTEH MRKLDAIVRE LQNPATPKEA TNYYVEYGG FEKVFKVPLV MPQEHLRCLA LDECHGVAAN GNGLALADKI VQSMSGLFRQ KHAFDVLLVY LPASWKKCFE Y DGFDLHDR IKAKVAPLNL PIQIINDTAL TRQCRANVMW GVSVALYAKA GGIPWKLADW DKDEAYIGLS YAIKKNAEGQ EY TTCCSQV FDPDGTGFEF VAYDTREFIT DRKGNPYLSY QEMQSVLSKS LHLYQSSHNG RMPRKIFIHK TTHFTEDEIQ GAF DSFSSS TEIELVQIIQ STNWYGLKVD GKKGDKPVAP ASYPVDRGLY QPLTESECLL WTQGSVMGVN QQNPGQPVFK EAAL TPLPN PIMLRRFSGN GGWHATCSSI LALTKVDWNN NTLYKKLPVT LVYSQVFADV VKQTPEIVNE IYDYRFFM UniProtKB:  Piwi domain protein Piwi domain protein |
-Macromolecule #3: NICOTINAMIDE-ADENINE-DINUCLEOTIDE
| Macromolecule | Name: NICOTINAMIDE-ADENINE-DINUCLEOTIDE / type: ligand / ID: 3 / Number of copies: 1 / Formula: NAD |
|---|---|
| Molecular weight | Theoretical: 663.425 Da |
| Chemical component information | 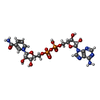 ChemComp-NAD: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 50 sec. | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Software | Name: EPU (ver. FEI) |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Initial model | Chain - Source name: AlphaFold / Chain - Initial model type: in silico model |
|---|---|
| Output model |  PDB-8jkz: |
 Movie
Movie Controller
Controller







 Z
Z Y
Y X
X


























