+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Acyl-ACP synthetase structure bound to C18:1-ACP | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Acyl-ACP synthetase / Tool enzyme /  CYTOSOLIC PROTEIN / CYTOSOLIC PROTEIN /  Ligase / TRANSPORT PROTEIN-CYTOSOLIC PROTEIN complex Ligase / TRANSPORT PROTEIN-CYTOSOLIC PROTEIN complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationKdo2-lipid A biosynthetic process / lipid A biosynthetic process /  acyl binding / acyl carrier activity / acyl binding / acyl carrier activity /  ligase activity / ligase activity /  cytosol cytosolSimilarity search - Function | |||||||||
| Biological species |   Vibrio harveyi (bacteria) / Vibrio harveyi (bacteria) /   Escherichia coli (E. coli) Escherichia coli (E. coli) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.63 Å cryo EM / Resolution: 2.63 Å | |||||||||
 Authors Authors | Huang H / Wang C / Chang S / Cui T / Xu Y / Zhang H / Zhou C / Zhang X / Feng Y | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Acyl-ACP synthetase structure bound to C18:1-ACP Authors: Huang H / Wang C / Chang S / Cui T / Xu Y / Zhang H / Zhou C / Zhang X / Feng Y | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35249.map.gz emd_35249.map.gz | 117.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35249-v30.xml emd-35249-v30.xml emd-35249.xml emd-35249.xml | 15.2 KB 15.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_35249.png emd_35249.png | 209.6 KB | ||
| Filedesc metadata |  emd-35249.cif.gz emd-35249.cif.gz | 5.6 KB | ||
| Others |  emd_35249_half_map_1.map.gz emd_35249_half_map_1.map.gz emd_35249_half_map_2.map.gz emd_35249_half_map_2.map.gz | 116 MB 116 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35249 http://ftp.pdbj.org/pub/emdb/structures/EMD-35249 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35249 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35249 | HTTPS FTP |
-Related structure data
| Related structure data |  8i8eMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_35249.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35249.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.014 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_35249_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_35249_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Acyl-ACP Synthetase bound to C18:1-ACP
| Entire | Name: Acyl-ACP Synthetase bound to C18:1-ACP |
|---|---|
| Components |
|
-Supramolecule #1: Acyl-ACP Synthetase bound to C18:1-ACP
| Supramolecule | Name: Acyl-ACP Synthetase bound to C18:1-ACP / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:   Vibrio harveyi (bacteria) Vibrio harveyi (bacteria) |
-Macromolecule #1: Acyl carrier protein
| Macromolecule | Name: Acyl carrier protein / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Molecular weight | Theoretical: 8.64546 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MSTIEERVKK IIGEQLGVKQ EEVTNNASFV EDLGADSLDT VELVMALEEE FDTEIPDEEA EKITTVQAAI DYINGHQA UniProtKB:  Acyl carrier protein Acyl carrier protein |
-Macromolecule #2: Acyl-acyl carrier protein synthetase
| Macromolecule | Name: Acyl-acyl carrier protein synthetase / type: protein_or_peptide / ID: 2 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Vibrio harveyi (bacteria) Vibrio harveyi (bacteria) |
| Molecular weight | Theoretical: 60.496258 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MNQYVNDPSN YQLLIKNLLF SPVAFNPEQE IVYANHRRHS YKTFHDRVRQ FANALTKMGV KKGDTVAVMD YDSHRYLECY FAIPMIGAK LHMINVRLSP EQILYTIDHA EDDIILIHEE FLPILDQIKG RIDTVTRYVV LRDDEECEYE RLLEQESTEY N FPDFDENT ...String: MNQYVNDPSN YQLLIKNLLF SPVAFNPEQE IVYANHRRHS YKTFHDRVRQ FANALTKMGV KKGDTVAVMD YDSHRYLECY FAIPMIGAK LHMINVRLSP EQILYTIDHA EDDIILIHEE FLPILDQIKG RIDTVTRYVV LRDDEECEYE RLLEQESTEY N FPDFDENT VATTFYTTGT TGFPKGVFFT HRQLVLHTMG ILSTIGTNAS QGRLHQGDIY MPITPMFHVH AWGLPYMATM LG VKQVYPG KYVPDVLLNL IEQEKVTFSH CVPTILHLLL SSPKSKAMDF SGWKVVIGGA ALPKALCKSA LERDIDVFAG YGM SETGPI LSIVQLTPEQ LELDVDQQAE YRSKTGKKVA LVEAYIVDED MNKLPHDGET AGEIVVRAPW LTPNYYKDNK NSKA LWRGG YLHTGDVAHI DDEGFIKITD RVKDMIKISG EWVSSLELED ILHQHQSVSE VAVIGMPHNK WGEVPLALVT LKEDA QVTE KELLGFAKDF INKGILAREA LLLKVKIVDE IAKTSVGKVD KKELRKLHL UniProtKB: Acyl-acyl carrier protein synthetase |
-Macromolecule #3: 4'-PHOSPHOPANTETHEINE
| Macromolecule | Name: 4'-PHOSPHOPANTETHEINE / type: ligand / ID: 3 / Number of copies: 6 / Formula: PNS |
|---|---|
| Molecular weight | Theoretical: 358.348 Da |
| Chemical component information |  ChemComp-PNS: |
-Macromolecule #4: ADENOSINE MONOPHOSPHATE
| Macromolecule | Name: ADENOSINE MONOPHOSPHATE / type: ligand / ID: 4 / Number of copies: 6 / Formula: AMP |
|---|---|
| Molecular weight | Theoretical: 347.221 Da |
| Chemical component information |  ChemComp-AMP: |
-Macromolecule #5: OLEIC ACID
| Macromolecule | Name: OLEIC ACID / type: ligand / ID: 5 / Number of copies: 6 / Formula: OLA |
|---|---|
| Molecular weight | Theoretical: 282.461 Da |
| Chemical component information | 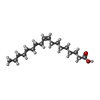 ChemComp-OLA: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.5 µm Bright-field microscopy / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.5 µm |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.63 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 299233 |
 Movie
Movie Controller
Controller






 Z
Z Y
Y X
X

















