[English] 日本語
 Yorodumi
Yorodumi- EMDB-35104: Structure of beta-arrestin1 in complex with a phosphopeptide corr... -
+ Open data
Open data
- Basic information
Basic information
| Entry | 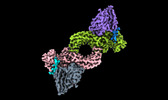 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of beta-arrestin1 in complex with a phosphopeptide corresponding to the human C5a anaphylatoxin chemotactic receptor 1, C5aR1 (Local refine) | |||||||||
 Map data Map data | Full map | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology information V2 vasopressin receptor binding / V2 vasopressin receptor binding /  alpha-1A adrenergic receptor binding / alpha-1A adrenergic receptor binding /  follicle-stimulating hormone receptor binding / sensory perception of touch / complement component C5a signaling pathway / presynapse organization / follicle-stimulating hormone receptor binding / sensory perception of touch / complement component C5a signaling pathway / presynapse organization /  regulation of tau-protein kinase activity / G alpha (s) signalling events / regulation of tau-protein kinase activity / G alpha (s) signalling events /  alpha-1B adrenergic receptor binding / complement component C5a receptor activity ... alpha-1B adrenergic receptor binding / complement component C5a receptor activity ... V2 vasopressin receptor binding / V2 vasopressin receptor binding /  alpha-1A adrenergic receptor binding / alpha-1A adrenergic receptor binding /  follicle-stimulating hormone receptor binding / sensory perception of touch / complement component C5a signaling pathway / presynapse organization / follicle-stimulating hormone receptor binding / sensory perception of touch / complement component C5a signaling pathway / presynapse organization /  regulation of tau-protein kinase activity / G alpha (s) signalling events / regulation of tau-protein kinase activity / G alpha (s) signalling events /  alpha-1B adrenergic receptor binding / complement component C5a receptor activity / follicle-stimulating hormone signaling pathway / protein phosphorylated amino acid binding / alpha-1B adrenergic receptor binding / complement component C5a receptor activity / follicle-stimulating hormone signaling pathway / protein phosphorylated amino acid binding /  angiotensin receptor binding / Lysosome Vesicle Biogenesis / AP-2 adaptor complex binding / response to peptidoglycan / Golgi Associated Vesicle Biogenesis / MAP2K and MAPK activation / Ub-specific processing proteases / positive regulation of smooth muscle cell apoptotic process / sensory perception of chemical stimulus / negative regulation of interleukin-8 production / Cargo recognition for clathrin-mediated endocytosis / angiotensin receptor binding / Lysosome Vesicle Biogenesis / AP-2 adaptor complex binding / response to peptidoglycan / Golgi Associated Vesicle Biogenesis / MAP2K and MAPK activation / Ub-specific processing proteases / positive regulation of smooth muscle cell apoptotic process / sensory perception of chemical stimulus / negative regulation of interleukin-8 production / Cargo recognition for clathrin-mediated endocytosis /  Clathrin-mediated endocytosis / clathrin adaptor activity / complement receptor mediated signaling pathway / regulation of G protein-coupled receptor signaling pathway / arrestin family protein binding / G protein-coupled receptor internalization / response to morphine / positive regulation of neutrophil chemotaxis / Thrombin signalling through proteinase activated receptors (PARs) / mitogen-activated protein kinase kinase binding / positive regulation of Rho protein signal transduction / Clathrin-mediated endocytosis / clathrin adaptor activity / complement receptor mediated signaling pathway / regulation of G protein-coupled receptor signaling pathway / arrestin family protein binding / G protein-coupled receptor internalization / response to morphine / positive regulation of neutrophil chemotaxis / Thrombin signalling through proteinase activated receptors (PARs) / mitogen-activated protein kinase kinase binding / positive regulation of Rho protein signal transduction /  clathrin binding / positive regulation of macrophage chemotaxis / clathrin binding / positive regulation of macrophage chemotaxis /  stress fiber assembly / negative regulation of Notch signaling pathway / amyloid-beta clearance / stress fiber assembly / negative regulation of Notch signaling pathway / amyloid-beta clearance /  pseudopodium / positive regulation of insulin secretion involved in cellular response to glucose stimulus / negative regulation of interleukin-6 production / cysteine-type endopeptidase inhibitor activity involved in apoptotic process / positive regulation of receptor internalization / pseudopodium / positive regulation of insulin secretion involved in cellular response to glucose stimulus / negative regulation of interleukin-6 production / cysteine-type endopeptidase inhibitor activity involved in apoptotic process / positive regulation of receptor internalization /  phototransduction / phototransduction /  : / positive regulation of vascular endothelial growth factor production / cellular defense response / : / positive regulation of vascular endothelial growth factor production / cellular defense response /  clathrin-coated pit / negative regulation of protein ubiquitination / clathrin-coated pit / negative regulation of protein ubiquitination /  insulin-like growth factor receptor binding / insulin-like growth factor receptor binding /  visual perception / Peptide ligand-binding receptors / visual perception / Peptide ligand-binding receptors /  GTPase activator activity / GTPase activator activity /  neutrophil chemotaxis / secretory granule membrane / negative regulation of protein phosphorylation / positive regulation of protein ubiquitination / neutrophil chemotaxis / secretory granule membrane / negative regulation of protein phosphorylation / positive regulation of protein ubiquitination /  Regulation of Complement cascade / positive regulation of epithelial cell proliferation / G protein-coupled receptor activity / G protein-coupled receptor binding / astrocyte activation / nuclear estrogen receptor binding / Regulation of Complement cascade / positive regulation of epithelial cell proliferation / G protein-coupled receptor activity / G protein-coupled receptor binding / astrocyte activation / nuclear estrogen receptor binding /  phosphoprotein binding / microglial cell activation / mRNA transcription by RNA polymerase II / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / negative regulation of ERK1 and ERK2 cascade / phosphoprotein binding / microglial cell activation / mRNA transcription by RNA polymerase II / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / negative regulation of ERK1 and ERK2 cascade /  cognition / cognition /  endocytosis / positive regulation of angiogenesis / endocytosis / positive regulation of angiogenesis /  chemotaxis / chemotaxis /  protein transport / apical part of cell / positive regulation of peptidyl-serine phosphorylation / phospholipase C-activating G protein-coupled receptor signaling pathway / positive regulation of cytosolic calcium ion concentration / G alpha (i) signalling events / cytoplasmic vesicle / ubiquitin-dependent protein catabolic process / protein transport / apical part of cell / positive regulation of peptidyl-serine phosphorylation / phospholipase C-activating G protein-coupled receptor signaling pathway / positive regulation of cytosolic calcium ion concentration / G alpha (i) signalling events / cytoplasmic vesicle / ubiquitin-dependent protein catabolic process /  postsynaptic membrane / basolateral plasma membrane / proteasome-mediated ubiquitin-dependent protein catabolic process / regulation of apoptotic process / negative regulation of neuron apoptotic process / transmembrane transporter binding / postsynaptic membrane / basolateral plasma membrane / proteasome-mediated ubiquitin-dependent protein catabolic process / regulation of apoptotic process / negative regulation of neuron apoptotic process / transmembrane transporter binding /  dendritic spine / positive regulation of MAPK cascade / dendritic spine / positive regulation of MAPK cascade /  transcription coactivator activity / positive regulation of ERK1 and ERK2 cascade / protein ubiquitination / transcription coactivator activity / positive regulation of ERK1 and ERK2 cascade / protein ubiquitination /  endosome / defense response to Gram-positive bacterium / response to xenobiotic stimulus / endosome / defense response to Gram-positive bacterium / response to xenobiotic stimulus /  immune response / immune response /  inflammatory response / positive regulation of protein phosphorylation / G protein-coupled receptor signaling pathway / inflammatory response / positive regulation of protein phosphorylation / G protein-coupled receptor signaling pathway /  signaling receptor binding signaling receptor bindingSimilarity search - Function | |||||||||
| Biological species |   Rattus norvegicus (Norway rat) / Rattus norvegicus (Norway rat) /   Homo sapiens (human) / Homo sapiens (human) /   Mus musculus (house mouse) Mus musculus (house mouse) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.26 Å cryo EM / Resolution: 3.26 Å | |||||||||
 Authors Authors | Maharana J / Sarma P / Yadav MK / Banerjee R / Shukla AK | |||||||||
| Funding support |  India, 1 items India, 1 items
| |||||||||
 Citation Citation |  Journal: Mol.Cell / Year: 2023 Journal: Mol.Cell / Year: 2023Title: Structure of beta-arrestin in complex with a phosphopeptide Authors: Maharana J / Sarma P / Yadav MK / Banerjee R / Shukla AK | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35104.map.gz emd_35104.map.gz | 86 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35104-v30.xml emd-35104-v30.xml emd-35104.xml emd-35104.xml | 21 KB 21 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_35104_fsc.xml emd_35104_fsc.xml | 9.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_35104.png emd_35104.png | 46 KB | ||
| Others |  emd_35104_half_map_1.map.gz emd_35104_half_map_1.map.gz emd_35104_half_map_2.map.gz emd_35104_half_map_2.map.gz | 84.3 MB 84.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35104 http://ftp.pdbj.org/pub/emdb/structures/EMD-35104 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35104 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35104 | HTTPS FTP |
-Related structure data
| Related structure data |  8i0nMC  8go8C  8gocC  8gooC  8gp3C  8i0qC  8i0zC  8i10C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_35104.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35104.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Full map | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.3667 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half map B
| File | emd_35104_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map A
| File | emd_35104_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Peptide 1 bound beta-arrestin1 in complex with Fab30 - Local refine
| Entire | Name: Peptide 1 bound beta-arrestin1 in complex with Fab30 - Local refine |
|---|---|
| Components |
|
-Supramolecule #1: Peptide 1 bound beta-arrestin1 in complex with Fab30 - Local refine
| Supramolecule | Name: Peptide 1 bound beta-arrestin1 in complex with Fab30 - Local refine type: complex / ID: 1 / Chimera: Yes / Parent: 0 / Macromolecule list: all |
|---|---|
| Molecular weight | Theoretical: 190 KDa |
-Supramolecule #2: beta-arrestin1
| Supramolecule | Name: beta-arrestin1 / type: complex / ID: 2 / Chimera: Yes / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat) |
-Supramolecule #3: C5a anaphylatoxin chemotactic receptor 1
| Supramolecule | Name: C5a anaphylatoxin chemotactic receptor 1 / type: complex / ID: 3 / Chimera: Yes / Parent: 1 / Macromolecule list: #4 / Details: Chemically synthesized |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #4: Fab30
| Supramolecule | Name: Fab30 / type: complex / ID: 4 / Chimera: Yes / Parent: 1 / Macromolecule list: #2-#3 |
|---|---|
| Source (natural) | Organism:   Mus musculus (house mouse) Mus musculus (house mouse) |
-Macromolecule #1: Beta-arrestin-1
| Macromolecule | Name: Beta-arrestin-1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat) |
| Molecular weight | Theoretical: 47.088508 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MGDKGTRVFK KASPNGKLTV YLGKRDFVDH IDLVDPVDGV VLVDPEYLKE RRVYVTLTCA FRYGREDLDV LGLTFRKDLF VANVQSFPP APEDKKPLTR LQERLIKKLG EHAYPFTFEI PPNLPCSVTL QPGPEDTGKA CGVDYEVKAF CAENLEEKIH K RNSVRLVI ...String: MGDKGTRVFK KASPNGKLTV YLGKRDFVDH IDLVDPVDGV VLVDPEYLKE RRVYVTLTCA FRYGREDLDV LGLTFRKDLF VANVQSFPP APEDKKPLTR LQERLIKKLG EHAYPFTFEI PPNLPCSVTL QPGPEDTGKA CGVDYEVKAF CAENLEEKIH K RNSVRLVI RKVQYAPERP GPQPTAETTR QFLMSDKPLH LEASLDKEIY YHGEPISVNV HVTNNTNKTV KKIKISVRQY AD ICLFNTA QYKCPVAMEE ADDTVAPSST FCKVYTLTPF LANNREKRGL ALDGKLKHED TNLASSTLLR EGANREILGI IVS YKVKVK LVVSRGGLLG DLASSDVAVE LPFTLMHPKP KEEPPHREVP ESETPVDTNL IELDTNDDDI VFEDFARQRL KGMK DDKDE EDDGTGSPHL NNR |
-Macromolecule #2: Fab30 heavy chain
| Macromolecule | Name: Fab30 heavy chain / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Mus musculus (house mouse) Mus musculus (house mouse) |
| Molecular weight | Theoretical: 25.512354 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: EISEVQLVES GGGLVQPGGS LRLSCAASGF NVYSSSIHWV RQAPGKGLEW VASISSYYGY TYYADSVKGR FTISADTSKN TAYLQMNSL RAEDTAVYYC ARSRQFWYSG LDYWGQGTLV TVSSASTKGP SVFPLAPSSK STSGGTAALG CLVKDYFPEP V TVSWNSGA ...String: EISEVQLVES GGGLVQPGGS LRLSCAASGF NVYSSSIHWV RQAPGKGLEW VASISSYYGY TYYADSVKGR FTISADTSKN TAYLQMNSL RAEDTAVYYC ARSRQFWYSG LDYWGQGTLV TVSSASTKGP SVFPLAPSSK STSGGTAALG CLVKDYFPEP V TVSWNSGA LTSGVHTFPA VLQSSGLYSL SSVVTVPSSS LGTQTYICNV NHKPSNTKVD KKVEPKSCDK THHHHHHHH |
-Macromolecule #3: Fab30 light chain
| Macromolecule | Name: Fab30 light chain / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Mus musculus (house mouse) Mus musculus (house mouse) |
| Molecular weight | Theoretical: 23.435064 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: SDIQMTQSPS SLSASVGDRV TITCRASQSV SSAVAWYQQK PGKAPKLLIY SASSLYSGVP SRFSGSRSGT DFTLTISSLQ PEDFATYYC QQYKYVPVTF GQGTKVEIKR TVAAPSVFIF PPSDSQLKSG TASVVCLLNN FYPREAKVQW KVDNALQSGN S QESVTEQD ...String: SDIQMTQSPS SLSASVGDRV TITCRASQSV SSAVAWYQQK PGKAPKLLIY SASSLYSGVP SRFSGSRSGT DFTLTISSLQ PEDFATYYC QQYKYVPVTF GQGTKVEIKR TVAAPSVFIF PPSDSQLKSG TASVVCLLNN FYPREAKVQW KVDNALQSGN S QESVTEQD SKDSTYSLSS TLTLSKADYE KHKVYACEVT HQGLSSPVTK SFNRGEC |
-Macromolecule #4: C5a anaphylatoxin chemotactic receptor 1
| Macromolecule | Name: C5a anaphylatoxin chemotactic receptor 1 / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 2.69834 KDa |
| Sequence | String: E(SEP)K(SEP)F(TPO)R(SEP)(TPO)V D(TPO)MAQKTQAV |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 Component:
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 283.15 K / Instrument: LEICA EM GP / Details: Blotted for 3 seconds before plunging.. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 165000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 165000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number real images: 6212 / Average electron dose: 56.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








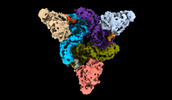
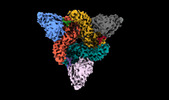















 Z
Z Y
Y X
X



















