[English] 日本語
 Yorodumi
Yorodumi- EMDB-34476: Cryo-EM structure of the transcription activation complex NtcA-TAC -
+ Open data
Open data
- Basic information
Basic information
| Entry | 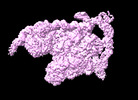 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the transcription activation complex NtcA-TAC | |||||||||||||||
 Map data Map data | ||||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | Transcription activation complex /  TRANSCRIPTION TRANSCRIPTION | |||||||||||||||
| Function / homology |  Function and homology information Function and homology information sigma factor activity / sigma factor activity /  DNA-directed RNA polymerase complex / DNA-templated transcription initiation / DNA-directed RNA polymerase complex / DNA-templated transcription initiation /  ribonucleoside binding / DNA-directed 5'-3' RNA polymerase activity / ribonucleoside binding / DNA-directed 5'-3' RNA polymerase activity /  DNA-directed RNA polymerase / DNA-directed RNA polymerase /  protein dimerization activity / DNA-binding transcription factor activity / DNA-templated transcription / magnesium ion binding ... protein dimerization activity / DNA-binding transcription factor activity / DNA-templated transcription / magnesium ion binding ... sigma factor activity / sigma factor activity /  DNA-directed RNA polymerase complex / DNA-templated transcription initiation / DNA-directed RNA polymerase complex / DNA-templated transcription initiation /  ribonucleoside binding / DNA-directed 5'-3' RNA polymerase activity / ribonucleoside binding / DNA-directed 5'-3' RNA polymerase activity /  DNA-directed RNA polymerase / DNA-directed RNA polymerase /  protein dimerization activity / DNA-binding transcription factor activity / DNA-templated transcription / magnesium ion binding / protein dimerization activity / DNA-binding transcription factor activity / DNA-templated transcription / magnesium ion binding /  DNA binding / zinc ion binding / DNA binding / zinc ion binding /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | |||||||||||||||
| Biological species |   Anabaena (bacteria) Anabaena (bacteria) | |||||||||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.6 Å cryo EM / Resolution: 3.6 Å | |||||||||||||||
 Authors Authors | Han SJ / Jiang YL / You LL / Shen LQ / Wu XX / Yang F / Kong WW / Chen ZP / Zhang Y / Zhou CZ | |||||||||||||||
| Funding support |  China, 4 items China, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2024 Journal: Nat Struct Mol Biol / Year: 2024Title: DNA looping mediates cooperative transcription activation. Authors: Shu-Jing Han / Yong-Liang Jiang / Lin-Lin You / Li-Qiang Shen / Xiaoxian Wu / Feng Yang / Ning Cui / Wen-Wen Kong / Hui Sun / Ke Zhou / Hui-Chao Meng / Zhi-Peng Chen / Yuxing Chen / Yu Zhang / Cong-Zhao Zhou /  Abstract: Transcription factors respond to multilevel stimuli and co-occupy promoter regions of target genes to activate RNA polymerase (RNAP) in a cooperative manner. To decipher the molecular mechanism, here ...Transcription factors respond to multilevel stimuli and co-occupy promoter regions of target genes to activate RNA polymerase (RNAP) in a cooperative manner. To decipher the molecular mechanism, here we report two cryo-electron microscopy structures of Anabaena transcription activation complexes (TACs): NtcA-TAC composed of RNAP holoenzyme, promoter and a global activator NtcA, and NtcA-NtcB-TAC comprising an extra context-specific regulator, NtcB. Structural analysis showed that NtcA binding makes the promoter DNA bend by ∼50°, which facilitates RNAP to contact NtcB at the distal upstream NtcB box. The sequential binding of NtcA and NtcB induces looping back of promoter DNA towards RNAP, enabling the assembly of a fully activated TAC bound with two activators. Together with biochemical assays, we propose a 'DNA looping' mechanism of cooperative transcription activation in bacteria. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34476.map.gz emd_34476.map.gz | 80.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34476-v30.xml emd-34476-v30.xml emd-34476.xml emd-34476.xml | 31.7 KB 31.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_34476_fsc.xml emd_34476_fsc.xml | 10.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_34476.png emd_34476.png | 93.5 KB | ||
| Filedesc metadata |  emd-34476.cif.gz emd-34476.cif.gz | 9.7 KB | ||
| Others |  emd_34476_half_map_1.map.gz emd_34476_half_map_1.map.gz emd_34476_half_map_2.map.gz emd_34476_half_map_2.map.gz | 80.8 MB 80.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34476 http://ftp.pdbj.org/pub/emdb/structures/EMD-34476 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34476 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34476 | HTTPS FTP |
-Related structure data
| Related structure data |  8h40MC  8h3vC  8h3zC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_34476.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34476.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_34476_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_34476_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : transcription activation complex with the global regulator NtcA
+Supramolecule #1: transcription activation complex with the global regulator NtcA
+Macromolecule #1: DNA (125-MER)
+Macromolecule #2: DNA (125-MER)
+Macromolecule #3: DNA-directed RNA polymerase subunit beta
+Macromolecule #4: DNA-directed RNA polymerase subunit beta'
+Macromolecule #5: DNA-directed RNA polymerase subunit alpha
+Macromolecule #6: DNA-directed RNA polymerase subunit gamma
+Macromolecule #7: DNA-directed RNA polymerase subunit omega
+Macromolecule #8: RNA polymerase sigma factor SigA
+Macromolecule #9: NtcA
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 15 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
Details: 10 mM HEPES pH 7.5, 100 mM KCl, 5 mM MgCl2 and 2 mM DTT | |||||||||||||||
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 120 sec. / Pretreatment - Atmosphere: OTHER | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK I |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Details | Preliminary grid screening was performed manually. |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 1462 / Average electron dose: 50.0 e/Å2 Details: Images were collected in movie-mode at 32 frames per second. |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-8h40: |
 Movie
Movie Controller
Controller








 Z
Z Y
Y X
X



















