[English] 日本語
 Yorodumi
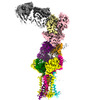
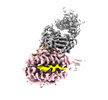
Yorodumi- EMDB-28572: CryoEM structure of PN45428 TCR-CD3 in complex with HLA-A2 MAGEA4 -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of PN45428 TCR-CD3 in complex with HLA-A2 MAGEA4 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | TCR /  receptor / receptor /  membrane protein / CD3 / MHC / HLA / membrane protein / CD3 / MHC / HLA /  IMMUNE SYSTEM IMMUNE SYSTEM | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of lymphocyte apoptotic process / gamma-delta T cell receptor complex /  Fc-gamma receptor III complex / T cell anergy / positive regulation of T cell anergy / positive regulation of cell-cell adhesion mediated by integrin / Fc-gamma receptor signaling pathway / CD4-positive, alpha-beta T cell proliferation / gamma-delta T cell activation / negative thymic T cell selection ...regulation of lymphocyte apoptotic process / gamma-delta T cell receptor complex / Fc-gamma receptor III complex / T cell anergy / positive regulation of T cell anergy / positive regulation of cell-cell adhesion mediated by integrin / Fc-gamma receptor signaling pathway / CD4-positive, alpha-beta T cell proliferation / gamma-delta T cell activation / negative thymic T cell selection ...regulation of lymphocyte apoptotic process / gamma-delta T cell receptor complex /  Fc-gamma receptor III complex / T cell anergy / positive regulation of T cell anergy / positive regulation of cell-cell adhesion mediated by integrin / Fc-gamma receptor signaling pathway / CD4-positive, alpha-beta T cell proliferation / gamma-delta T cell activation / negative thymic T cell selection / positive regulation of CD4-positive, alpha-beta T cell proliferation / positive regulation of protein localization to cell surface / alpha-beta T cell receptor complex / positive thymic T cell selection / signal complex assembly / Nef and signal transduction / positive regulation of cell-matrix adhesion / Fc-gamma receptor III complex / T cell anergy / positive regulation of T cell anergy / positive regulation of cell-cell adhesion mediated by integrin / Fc-gamma receptor signaling pathway / CD4-positive, alpha-beta T cell proliferation / gamma-delta T cell activation / negative thymic T cell selection / positive regulation of CD4-positive, alpha-beta T cell proliferation / positive regulation of protein localization to cell surface / alpha-beta T cell receptor complex / positive thymic T cell selection / signal complex assembly / Nef and signal transduction / positive regulation of cell-matrix adhesion /  T cell receptor complex / smoothened signaling pathway / establishment or maintenance of cell polarity / Translocation of ZAP-70 to Immunological synapse / Phosphorylation of CD3 and TCR zeta chains / dendrite development / Generation of second messenger molecules / alpha-beta T cell activation / T cell receptor complex / smoothened signaling pathway / establishment or maintenance of cell polarity / Translocation of ZAP-70 to Immunological synapse / Phosphorylation of CD3 and TCR zeta chains / dendrite development / Generation of second messenger molecules / alpha-beta T cell activation /  immunological synapse / FCGR activation / antigen processing and presentation / PD-1 signaling / positive regulation of interleukin-4 production / Role of phospholipids in phagocytosis / positive regulation of cell cycle / negative regulation of smoothened signaling pathway / positive regulation of calcium-mediated signaling / positive regulation of T cell proliferation / immunological synapse / FCGR activation / antigen processing and presentation / PD-1 signaling / positive regulation of interleukin-4 production / Role of phospholipids in phagocytosis / positive regulation of cell cycle / negative regulation of smoothened signaling pathway / positive regulation of calcium-mediated signaling / positive regulation of T cell proliferation /  protein tyrosine kinase binding / T cell costimulation / positive regulation of interleukin-2 production / protein tyrosine kinase binding / T cell costimulation / positive regulation of interleukin-2 production /  T cell receptor binding / cerebellum development / T cell receptor binding / cerebellum development /  T cell activation / FCGR3A-mediated IL10 synthesis / positive regulation of ferrous iron binding / positive regulation of transferrin receptor binding / positive regulation of receptor binding / early endosome lumen / Nef mediated downregulation of MHC class I complex cell surface expression / DAP12 interactions / negative regulation of receptor binding / calcium-mediated signaling / apoptotic signaling pathway / lumenal side of endoplasmic reticulum membrane / Endosomal/Vacuolar pathway / Antigen Presentation: Folding, assembly and peptide loading of class I MHC / FCGR3A-mediated phagocytosis / clathrin-coated endocytic vesicle membrane / antigen processing and presentation of exogenous protein antigen via MHC class Ib, TAP-dependent / cellular response to iron(III) ion / negative regulation of forebrain neuron differentiation / ER to Golgi transport vesicle membrane / peptide antigen assembly with MHC class I protein complex / response to molecule of bacterial origin / T cell activation / FCGR3A-mediated IL10 synthesis / positive regulation of ferrous iron binding / positive regulation of transferrin receptor binding / positive regulation of receptor binding / early endosome lumen / Nef mediated downregulation of MHC class I complex cell surface expression / DAP12 interactions / negative regulation of receptor binding / calcium-mediated signaling / apoptotic signaling pathway / lumenal side of endoplasmic reticulum membrane / Endosomal/Vacuolar pathway / Antigen Presentation: Folding, assembly and peptide loading of class I MHC / FCGR3A-mediated phagocytosis / clathrin-coated endocytic vesicle membrane / antigen processing and presentation of exogenous protein antigen via MHC class Ib, TAP-dependent / cellular response to iron(III) ion / negative regulation of forebrain neuron differentiation / ER to Golgi transport vesicle membrane / peptide antigen assembly with MHC class I protein complex / response to molecule of bacterial origin /  regulation of erythrocyte differentiation / regulation of iron ion transport / MHC class I peptide loading complex / HFE-transferrin receptor complex / T cell mediated cytotoxicity / cellular response to iron ion / Regulation of actin dynamics for phagocytic cup formation / antigen processing and presentation of endogenous peptide antigen via MHC class I / positive regulation of T cell cytokine production / MHC class I protein complex / multicellular organismal-level iron ion homeostasis / regulation of erythrocyte differentiation / regulation of iron ion transport / MHC class I peptide loading complex / HFE-transferrin receptor complex / T cell mediated cytotoxicity / cellular response to iron ion / Regulation of actin dynamics for phagocytic cup formation / antigen processing and presentation of endogenous peptide antigen via MHC class I / positive regulation of T cell cytokine production / MHC class I protein complex / multicellular organismal-level iron ion homeostasis /  SH3 domain binding / positive regulation of T cell mediated cytotoxicity / peptide antigen assembly with MHC class II protein complex / negative regulation of neurogenesis / MHC class II protein complex / positive regulation of receptor-mediated endocytosis / cellular response to nicotine / SH3 domain binding / positive regulation of T cell mediated cytotoxicity / peptide antigen assembly with MHC class II protein complex / negative regulation of neurogenesis / MHC class II protein complex / positive regulation of receptor-mediated endocytosis / cellular response to nicotine /  histone deacetylase binding / recycling endosome membrane / specific granule lumen / phagocytic vesicle membrane / peptide antigen binding / histone deacetylase binding / recycling endosome membrane / specific granule lumen / phagocytic vesicle membrane / peptide antigen binding /  cell surface receptor protein tyrosine kinase signaling pathway / positive regulation of cellular senescence / antigen processing and presentation of exogenous peptide antigen via MHC class II / Immunoregulatory interactions between a Lymphoid and a non-Lymphoid cell / positive regulation of immune response / Interferon gamma signaling / negative regulation of epithelial cell proliferation / Modulation by Mtb of host immune system / positive regulation of T cell activation / positive regulation of peptidyl-tyrosine phosphorylation / cell-cell junction / positive regulation of type II interferon production / sensory perception of smell / transmembrane signaling receptor activity / signaling receptor complex adaptor activity cell surface receptor protein tyrosine kinase signaling pathway / positive regulation of cellular senescence / antigen processing and presentation of exogenous peptide antigen via MHC class II / Immunoregulatory interactions between a Lymphoid and a non-Lymphoid cell / positive regulation of immune response / Interferon gamma signaling / negative regulation of epithelial cell proliferation / Modulation by Mtb of host immune system / positive regulation of T cell activation / positive regulation of peptidyl-tyrosine phosphorylation / cell-cell junction / positive regulation of type II interferon production / sensory perception of smell / transmembrane signaling receptor activity / signaling receptor complex adaptor activitySimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.25 Å cryo EM / Resolution: 3.25 Å | |||||||||
 Authors Authors | Saotome K / Franklin MC | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural analysis of cancer-relevant TCR-CD3 and peptide-MHC complexes by cryoEM. Authors: Kei Saotome / Drew Dudgeon / Kiersten Colotti / Michael J Moore / Jennifer Jones / Yi Zhou / Ashique Rafique / George D Yancopoulos / Andrew J Murphy / John C Lin / William C Olson / Matthew C Franklin /  Abstract: The recognition of antigenic peptide-MHC (pMHC) molecules by T-cell receptors (TCR) initiates the T-cell mediated immune response. Structural characterization is key for understanding the specificity ...The recognition of antigenic peptide-MHC (pMHC) molecules by T-cell receptors (TCR) initiates the T-cell mediated immune response. Structural characterization is key for understanding the specificity of TCR-pMHC interactions and informing the development of therapeutics. Despite the rapid rise of single particle cryoelectron microscopy (cryoEM), x-ray crystallography has remained the preferred method for structure determination of TCR-pMHC complexes. Here, we report cryoEM structures of two distinct full-length α/β TCR-CD3 complexes bound to their pMHC ligand, the cancer-testis antigen HLA-A2/MAGEA4 (230-239). We also determined cryoEM structures of pMHCs containing MAGEA4 (230-239) peptide and the closely related MAGEA8 (232-241) peptide in the absence of TCR, which provided a structural explanation for the MAGEA4 preference displayed by the TCRs. These findings provide insights into the TCR recognition of a clinically relevant cancer antigen and demonstrate the utility of cryoEM for high-resolution structural analysis of TCR-pMHC interactions. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28572.map.gz emd_28572.map.gz | 270.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28572-v30.xml emd-28572-v30.xml emd-28572.xml emd-28572.xml | 24.4 KB 24.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_28572.png emd_28572.png | 74.2 KB | ||
| Filedesc metadata |  emd-28572.cif.gz emd-28572.cif.gz | 7.2 KB | ||
| Others |  emd_28572_half_map_1.map.gz emd_28572_half_map_1.map.gz emd_28572_half_map_2.map.gz emd_28572_half_map_2.map.gz | 261.4 MB 261.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28572 http://ftp.pdbj.org/pub/emdb/structures/EMD-28572 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28572 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28572 | HTTPS FTP |
-Related structure data
| Related structure data |  8es9MC  8es7C  8es8C  8esaC  8esbC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_28572.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28572.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.85 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_28572_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_28572_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : PN45428 TCR-CD3 complex bound to HLA-A2 MAGEA4 (230-239) in the p...
+Supramolecule #1: PN45428 TCR-CD3 complex bound to HLA-A2 MAGEA4 (230-239) in the p...
+Macromolecule #1: PN45428 TCR alpha chain
+Macromolecule #2: PN45428 TCR beta chain
+Macromolecule #3: T-cell surface glycoprotein CD3 zeta chain
+Macromolecule #4: T-cell surface glycoprotein CD3 delta chain
+Macromolecule #5: T-cell surface glycoprotein CD3 epsilon chain
+Macromolecule #6: T-cell surface glycoprotein CD3 gamma chain
+Macromolecule #7: MHC class I antigen
+Macromolecule #8: Beta-2-microglobulin
+Macromolecule #9: Melanoma-associated antigen 4
+Macromolecule #12: 2-acetamido-2-deoxy-beta-D-glucopyranose
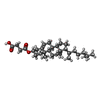
+Macromolecule #13: CHOLESTEROL HEMISUCCINATE
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.4000000000000001 µm Bright-field microscopy / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.4000000000000001 µm |
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.25 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 107308 |
 Movie
Movie Controller
Controller





















 Z
Z Y
Y X
X