[English] 日本語
 Yorodumi
Yorodumi- EMDB-27989: Octameric prenyltransferase domain of fusicoccadiene Synthase wit... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Octameric prenyltransferase domain of fusicoccadiene Synthase with C2 symmetry sans transiently associating cyclase domains | |||||||||
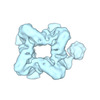
 Map data Map data | Structure of the octameric C-terminal prenyltransferase domain of fusicoccadiene synthase from Phomopsis amygdali (PaFS) at 3.73-angstrom resolution | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology information fusicocca-2,10(14)-diene synthase / alcohol biosynthetic process / mycotoxin biosynthetic process / geranylgeranyl diphosphate synthase / fusicocca-2,10(14)-diene synthase / alcohol biosynthetic process / mycotoxin biosynthetic process / geranylgeranyl diphosphate synthase /  farnesyltranstransferase activity / isoprenoid biosynthetic process / farnesyltranstransferase activity / isoprenoid biosynthetic process /  lyase activity / lyase activity /  metal ion binding metal ion bindingSimilarity search - Function | |||||||||
| Biological species |   Diaporthe amygdali (fungus) Diaporthe amygdali (fungus) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.73 Å cryo EM / Resolution: 3.73 Å | |||||||||
 Authors Authors | Faylo JL / van Eeuwen T / Christianson DW | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Biochemistry / Year: 2022 Journal: Biochemistry / Year: 2022Title: Transient Prenyltransferase-Cyclase Association in Fusicoccadiene Synthase, an Assembly-Line Terpene Synthase. Authors: Jacque L Faylo / Trevor van Eeuwen / Kushol Gupta / Kenji Murakami / David W Christianson /  Abstract: Fusicoccadiene synthase from the fungus (PaFS) is an assembly-line terpene synthase that catalyzes the first two steps in the biosynthesis of Fusiccocin A, a diterpene glycoside. The C-terminal ...Fusicoccadiene synthase from the fungus (PaFS) is an assembly-line terpene synthase that catalyzes the first two steps in the biosynthesis of Fusiccocin A, a diterpene glycoside. The C-terminal prenyltransferase domain of PaFS catalyzes the condensation of one molecule of C dimethylallyl diphosphate and three molecules of C isopentenyl diphosphate to form C geranylgeranyl diphosphate, which then transits to the cyclase domain for cyclization to form fusicoccadiene. Previous structural studies of PaFS using electron microscopy (EM) revealed a central octameric prenyltransferase core with eight cyclase domains tethered in random distal positions through flexible 70-residue linkers. However, proximal prenyltransferase-cyclase configurations could be captured by covalent cross-linking and observed by cryo-EM and mass spectrometry. Here, we use cryo-EM to show that proximally configured prenyltransferase-cyclase complexes are observable even in the absence of covalent cross-linking; moreover, such complexes can involve multiple cyclase domains. A conserved basic patch on the prenyltransferase domain comprises the primary touchpoint with the cyclase domain. These results support a model for transient prenyltransferase-cyclase association in which the cyclase domains of PaFS are in facile equilibrium between proximal associated and random distal positions relative to the central prenyltransferase octamer. The results of biophysical measurements using small-angle X-ray scattering, analytical ultracentrifugation, dynamic light scattering, and size-exclusion chromatography in-line with multi-angle light scattering are consistent with this model. This model accordingly provides a framework for understanding substrate transit between the prenyltransferase and cyclase domains as well as the cooperativity observed for geranylgeranyl diphosphate cyclization. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27989.map.gz emd_27989.map.gz | 189.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27989-v30.xml emd-27989-v30.xml emd-27989.xml emd-27989.xml | 17.2 KB 17.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_27989_fsc.xml emd_27989_fsc.xml | 12.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_27989.png emd_27989.png | 204.8 KB | ||
| Masks |  emd_27989_msk_1.map emd_27989_msk_1.map | 216 MB |  Mask map Mask map | |
| Others |  emd_27989_additional_1.map.gz emd_27989_additional_1.map.gz emd_27989_half_map_1.map.gz emd_27989_half_map_1.map.gz emd_27989_half_map_2.map.gz emd_27989_half_map_2.map.gz | 106.4 MB 200.4 MB 200.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27989 http://ftp.pdbj.org/pub/emdb/structures/EMD-27989 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27989 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27989 | HTTPS FTP |
-Related structure data
| Related structure data |  8eaxMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27989.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27989.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Structure of the octameric C-terminal prenyltransferase domain of fusicoccadiene synthase from Phomopsis amygdali (PaFS) at 3.73-angstrom resolution | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_27989_msk_1.map emd_27989_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Unsharpened map generated in non-uniform refinement of the...
| File | emd_27989_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened map generated in non-uniform refinement of the PaFS prenyltransferase octamer | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half-map 1 of the octameric C-terminal prenyltransferase domain...
| File | emd_27989_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map 1 of the octameric C-terminal prenyltransferase domain of PaFS at 3.73-angstrom resolution | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half-map 2 of the octameric C-terminal prenyltransferase domain...
| File | emd_27989_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map 2 of the octameric C-terminal prenyltransferase domain of PaFS at 3.73-angstrom resolution | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Octamer of the prenylstransferase domain of fusicoccadiene syntha...
| Entire | Name: Octamer of the prenylstransferase domain of fusicoccadiene synthase from Phomopsis amygdali (PaFS) |
|---|---|
| Components |
|
-Supramolecule #1: Octamer of the prenylstransferase domain of fusicoccadiene syntha...
| Supramolecule | Name: Octamer of the prenylstransferase domain of fusicoccadiene synthase from Phomopsis amygdali (PaFS) type: complex / Chimera: Yes / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Diaporthe amygdali (fungus) Diaporthe amygdali (fungus) |
| Recombinant expression | Organism:   Escherichia coli BL21(DE3) (bacteria) Escherichia coli BL21(DE3) (bacteria) |
| Molecular weight | Experimental: 392 KDa |
-Macromolecule #1: Fusicoccadiene synthase
| Macromolecule | Name: Fusicoccadiene synthase / type: protein_or_peptide / ID: 1 / Number of copies: 8 / Enantiomer: LEVO / EC number:  fusicocca-2,10(14)-diene synthase fusicocca-2,10(14)-diene synthase |
|---|---|
| Source (natural) | Organism:   Diaporthe amygdali (fungus) Diaporthe amygdali (fungus) |
| Molecular weight | Theoretical: 83.927977 KDa |
| Recombinant expression | Organism:   Escherichia coli BL21(DE3) (bacteria) Escherichia coli BL21(DE3) (bacteria) |
| Sequence | String: MGSSHHHHHH SSGLVPRGSH MEFKYSEVVE PSTYYTEGLC EGIDVRKSKF TTLEDRGAIR AHEDWNKHIG PCREYRGTLG PRFSFISVA VPECIPERLE VISYANEFAF LHDDVTDHVG HDTGEVENDE MMTVFLEAAH TGAIDTSNKV DIRRAGKKRI Q SQLFLEML ...String: MGSSHHHHHH SSGLVPRGSH MEFKYSEVVE PSTYYTEGLC EGIDVRKSKF TTLEDRGAIR AHEDWNKHIG PCREYRGTLG PRFSFISVA VPECIPERLE VISYANEFAF LHDDVTDHVG HDTGEVENDE MMTVFLEAAH TGAIDTSNKV DIRRAGKKRI Q SQLFLEML AIDPECAKTT MKSWARFVEV GSSRQHETRF VELAKYIPYR IMDVGEMFWF GLVTFGLGLH IPDHELELCR EL MANAWIA VGLQNDIWSW PKERDAATLH GKDHVVNAIW VLMQEHQTDV DGAMQICRKL IVEYVAKYLE VIEATKNDES ISL DLRKYL DAMLYSISGN VVWSLECPRY NPDVSFNKTQ LEWMRQGLPS LESCPVLARS PEIDSDESAV SPTADESDST EDSL GSGSR QDSSLSTGLS LSPVHSNEGK DLQRVDTDHI FFEKAVLEAP YDYIASMPSK GVRDQFIDAL NDWLRVPDVK VGKIK DAVR VLHNSSLLLD DFQDNSPLRR GKPSTHNIFG SAQTVNTATY SIIKAIGQIM EFSAGESVQE VMNSIMILFQ GQAMDL FWT YNGHVPSEEE YYRMIDQKTG QLFSIATSLL LNAADNEIPR TKIQSCLHRL TRLLGRCFQI CDDYQNLVSA DYTKQKG FC EDLDEGKWSL ALIHMIHKQR SHMALLNVLS TGRKHGGMTL EQKQFVLDII EEEKSLDYTR SVMMDLHVQL RAEIGRIE I LLDSPNPAMR LLLELLRV |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm Bright-field microscopy / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 52.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



























 Z
Z Y
Y X
X