[English] 日本語
 Yorodumi
Yorodumi- EMDB-25585: Complex of GABA-A synaptic receptor with autoimmune antibody Fab175 -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Complex of GABA-A synaptic receptor with autoimmune antibody Fab175 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  autoimmunity / encephalitis / GABA / autoimmunity / encephalitis / GABA /  inhibitory / inhibitory /  MEMBRANE PROTEIN-IMMUNE SYSTEM complex MEMBRANE PROTEIN-IMMUNE SYSTEM complex | |||||||||
| Function / homology |  Function and homology information Function and homology information benzodiazepine receptor activity / benzodiazepine receptor activity /  GABA receptor complex / inhibitory extracellular ligand-gated monoatomic ion channel activity / GABA receptor activation / inner ear receptor cell development / GABA-gated chloride ion channel activity / cellular response to histamine / GABA receptor complex / inhibitory extracellular ligand-gated monoatomic ion channel activity / GABA receptor activation / inner ear receptor cell development / GABA-gated chloride ion channel activity / cellular response to histamine /  inhibitory synapse assembly / inhibitory synapse assembly /  GABA-A receptor activity / GABA-A receptor activity /  GABA-A receptor complex ... GABA-A receptor complex ... benzodiazepine receptor activity / benzodiazepine receptor activity /  GABA receptor complex / inhibitory extracellular ligand-gated monoatomic ion channel activity / GABA receptor activation / inner ear receptor cell development / GABA-gated chloride ion channel activity / cellular response to histamine / GABA receptor complex / inhibitory extracellular ligand-gated monoatomic ion channel activity / GABA receptor activation / inner ear receptor cell development / GABA-gated chloride ion channel activity / cellular response to histamine /  inhibitory synapse assembly / inhibitory synapse assembly /  GABA-A receptor activity / GABA-A receptor activity /  GABA-A receptor complex / GABA-A receptor complex /  innervation / postsynaptic specialization membrane / innervation / postsynaptic specialization membrane /  neurotransmitter receptor activity / neurotransmitter receptor activity /  synaptic transmission, GABAergic / gamma-aminobutyric acid signaling pathway / synaptic transmission, GABAergic / gamma-aminobutyric acid signaling pathway /  chloride channel activity / chloride channel activity /  adult behavior / cochlea development / Signaling by ERBB4 / adult behavior / cochlea development / Signaling by ERBB4 /  chloride channel complex / GABA-ergic synapse / regulation of postsynaptic membrane potential / chloride transmembrane transport / dendrite membrane / post-embryonic development / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / cytoplasmic vesicle membrane / chemical synaptic transmission / postsynapse / chloride channel complex / GABA-ergic synapse / regulation of postsynaptic membrane potential / chloride transmembrane transport / dendrite membrane / post-embryonic development / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / cytoplasmic vesicle membrane / chemical synaptic transmission / postsynapse /  postsynaptic membrane / neuron projection / postsynaptic membrane / neuron projection /  axon / axon /  synapse / extracellular exosome / synapse / extracellular exosome /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.0 Å cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Noviello CM / Hibbs RE / Kreye J / Teng J / Pruss H | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2022 Journal: Cell / Year: 2022Title: Structural mechanisms of GABA receptor autoimmune encephalitis. Authors: Colleen M Noviello / Jakob Kreye / Jinfeng Teng / Harald Prüss / Ryan E Hibbs /   Abstract: Autoantibodies targeting neuronal membrane proteins can cause encephalitis, seizures, and severe behavioral abnormalities. While antibodies for several neuronal targets have been identified, ...Autoantibodies targeting neuronal membrane proteins can cause encephalitis, seizures, and severe behavioral abnormalities. While antibodies for several neuronal targets have been identified, structural details on how they regulate function are unknown. Here we determined cryo-electron microscopy structures of antibodies derived from an encephalitis patient bound to the γ-aminobutyric acid type A (GABA) receptor. These antibodies induced severe encephalitis by directly inhibiting GABA function, resulting in nervous-system hyperexcitability. The structures reveal mechanisms of GABA inhibition and pathology. One antibody directly competes with a neurotransmitter and locks the receptor in a resting-like state. The second antibody targets the subunit interface involved in binding benzodiazepines and antagonizes diazepam potentiation. We identify key residues in these antibodies involved in specificity and affinity and confirm structure-based hypotheses for functional effects using electrophysiology. Together these studies define mechanisms of direct functional antagonism of neurotransmission underlying autoimmune encephalitis in a human patient. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25585.map.gz emd_25585.map.gz | 8.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25585-v30.xml emd-25585-v30.xml emd-25585.xml emd-25585.xml | 22.3 KB 22.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_25585.png emd_25585.png | 43.3 KB | ||
| Filedesc metadata |  emd-25585.cif.gz emd-25585.cif.gz | 6.7 KB | ||
| Others |  emd_25585_additional_1.map.gz emd_25585_additional_1.map.gz emd_25585_half_map_1.map.gz emd_25585_half_map_1.map.gz emd_25585_half_map_2.map.gz emd_25585_half_map_2.map.gz | 71.4 MB 30.9 MB 30.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25585 http://ftp.pdbj.org/pub/emdb/structures/EMD-25585 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25585 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25585 | HTTPS FTP |
-Related structure data
| Related structure data |  7t0zMC  7t0wC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25585.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25585.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.079 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_25585_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_25585_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_25585_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Complex between autoantibody Fab175 and the a2b2g2 GABA-A receptor
| Entire | Name: Complex between autoantibody Fab175 and the a2b2g2 GABA-A receptor |
|---|---|
| Components |
|
-Supramolecule #1: Complex between autoantibody Fab175 and the a2b2g2 GABA-A receptor
| Supramolecule | Name: Complex between autoantibody Fab175 and the a2b2g2 GABA-A receptor type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Gamma-aminobutyric acid receptor subunit beta-2
| Macromolecule | Name: Gamma-aminobutyric acid receptor subunit beta-2 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 39.40759 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QSVNDPSNMS LVKETVDRLL KGYDIRLRPD FGGPPVAVGM NIDIASIDMV SEVNMDYTLT MYFQQAWRDK RLSYNVIPLN LTLDNRVAD QLWVPDTYFL NDKKSFVHGV TVKNRMIRLH PDGTVLYGLR ITTTAACMMD LRRYPLDEQN CTLEIESYGY T TDDIEFYW ...String: QSVNDPSNMS LVKETVDRLL KGYDIRLRPD FGGPPVAVGM NIDIASIDMV SEVNMDYTLT MYFQQAWRDK RLSYNVIPLN LTLDNRVAD QLWVPDTYFL NDKKSFVHGV TVKNRMIRLH PDGTVLYGLR ITTTAACMMD LRRYPLDEQN CTLEIESYGY T TDDIEFYW RGDDNAVTGV TKIELPQFSI VDYKLITKKV VFSTGSYPRL SLSFKLKRNI GYFILQTYMP SILITILSWV SF WINYDAS AARVALGITT VLTMTTINTH LRETLPKIPY VKAIDMYLMG CFVFVFMALL EYALVNYIFF SQPARAAAID RWS RIFFPV VFSFFNIVYW LYYV UniProtKB: Gamma-aminobutyric acid receptor subunit beta-2, Gamma-aminobutyric acid receptor subunit beta-2 |
-Macromolecule #2: Gamma-aminobutyric acid receptor subunit alpha-1
| Macromolecule | Name: Gamma-aminobutyric acid receptor subunit alpha-1 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 39.831836 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QPSLQDELKD NTTVFTRILD RLLDGYDNRL RPGLGERVTE VKTDIFVTSF GPVSDHDMEY TIDVFFRQSW KDERLKFKGP MTVLRLNNL MASKIWTPDT FFHNGKKSVA HNMTMPNKLL RITEDGTLLY TMRLTVRAEC PMHLEDFPMD AHACPLKFGS Y AYTRAEVV ...String: QPSLQDELKD NTTVFTRILD RLLDGYDNRL RPGLGERVTE VKTDIFVTSF GPVSDHDMEY TIDVFFRQSW KDERLKFKGP MTVLRLNNL MASKIWTPDT FFHNGKKSVA HNMTMPNKLL RITEDGTLLY TMRLTVRAEC PMHLEDFPMD AHACPLKFGS Y AYTRAEVV YEWTREPARS VVVAEDGSRL NQYDLLGQTV DSGIVQSSTG EYVVMTTHFH LKRKIGYFVI QTYLPCIMTV IL SQVSFWL NRESVPARTV FGVTTVLTMT TLSISARNSL PKVAYATAMD WFIAVCYAFV FSALIEFATV NYFTKSQPAR AAK IDRLSR IAFPLLFGIF NLVYWATYLN R UniProtKB: Gamma-aminobutyric acid receptor subunit alpha-1, Gamma-aminobutyric acid receptor subunit alpha-1 |
-Macromolecule #3: Gamma-aminobutyric acid receptor subunit gamma-2
| Macromolecule | Name: Gamma-aminobutyric acid receptor subunit gamma-2 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 45.35466 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: WSHPQFEKGG GSGGGSGGSS AWSHPQFEKL EVLFQGPQKS DDDYEDYASN KTWVLTPKVP EGDVTVILNN LLEGYDNKLR PDIGVKPTL IHTDMYVNSI GPVNAINMEY TIDIFFAQTW YDRRLKFNST IKVLRLNSNM VGKIWIPDTF FRNSKKADAH W ITTPNRML ...String: WSHPQFEKGG GSGGGSGGSS AWSHPQFEKL EVLFQGPQKS DDDYEDYASN KTWVLTPKVP EGDVTVILNN LLEGYDNKLR PDIGVKPTL IHTDMYVNSI GPVNAINMEY TIDIFFAQTW YDRRLKFNST IKVLRLNSNM VGKIWIPDTF FRNSKKADAH W ITTPNRML RIWNDGRVLY TLRLTIDAEC QLQLHNFPMD EHSCPLEFSS YGYPREEIVY QWKRSSVEVG DTRSWRLYQF SF VGLRNTT EVVKTTSGDY VVMSVYFDLS RRMGYFTIQT YIPCTLIVVL SWVSFWINKD AVPARTSLGI TTVLTMTTLS TIA RKSLPK VSYVTAMDLF VSVCFIFVFS ALVEYGTLHY FVSSQPARAA KMDSYARIFF PTAFCLFNLV YWVSYLYL UniProtKB: Gamma-aminobutyric acid receptor subunit gamma-2, Gamma-aminobutyric acid receptor subunit gamma-2 |
-Macromolecule #4: Fab175 IgG1 heavy chain
| Macromolecule | Name: Fab175 IgG1 heavy chain / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 26.410365 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: EVQLLESGEG LVQPGGSLRL SCAASGFTFK TYAMSWVRQA PGKGLEWVSA ISGSGASTYY ADFVEGRFTI SRDNSKNTLY LQMNSLRAE DTAVYYCAKE PPAMVWFGGG YFDYWGQGTL VTVSSASTKG PSVFPLAPSS KSTSGGTAAL GCLVKDYFPE P VTVSWNSG ...String: EVQLLESGEG LVQPGGSLRL SCAASGFTFK TYAMSWVRQA PGKGLEWVSA ISGSGASTYY ADFVEGRFTI SRDNSKNTLY LQMNSLRAE DTAVYYCAKE PPAMVWFGGG YFDYWGQGTL VTVSSASTKG PSVFPLAPSS KSTSGGTAAL GCLVKDYFPE P VTVSWNSG ALTSGVHTFP AVLQSSGLYS LSSVVTVPSS SLGTQTYICN VNHKPSNTKV DKRVEPKSCD KTHDYKDDDD KH HHHHH |
-Macromolecule #5: Fab175 IgG1 light chain
| Macromolecule | Name: Fab175 IgG1 light chain / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 23.569117 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: EIVLTQSPAT LSLSPGERAT LSCRASQSVS SYLAWYQQKP GQAPRLLIYD ASNRATGIPA RFSGSGSGTD FTLTISSLEP EDFAVYYCQ QRSNWPPYSF GQGTKLEIKR TVAAPSVFIF PPSDEQLKSG TASVVCLLNN FYPREAKVQW KVDNALQSGN S QESVTEQD ...String: EIVLTQSPAT LSLSPGERAT LSCRASQSVS SYLAWYQQKP GQAPRLLIYD ASNRATGIPA RFSGSGSGTD FTLTISSLEP EDFAVYYCQ QRSNWPPYSF GQGTKLEIKR TVAAPSVFIF PPSDEQLKSG TASVVCLLNN FYPREAKVQW KVDNALQSGN S QESVTEQD SKDSTYSLSS TLTLSKADYE KHKVYACEVT HQGLSSPVTK SFNRGEC |
-Macromolecule #9: GAMMA-AMINO-BUTANOIC ACID
| Macromolecule | Name: GAMMA-AMINO-BUTANOIC ACID / type: ligand / ID: 9 / Number of copies: 2 / Formula: ABU |
|---|---|
| Molecular weight | Theoretical: 103.12 Da |
| Chemical component information |  ChemComp-ABU: |
-Macromolecule #10: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 10 / Number of copies: 2 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #11: alpha-D-mannopyranose
| Macromolecule | Name: alpha-D-mannopyranose / type: ligand / ID: 11 / Number of copies: 1 / Formula: MAN |
|---|---|
| Molecular weight | Theoretical: 180.156 Da |
| Chemical component information | 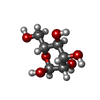 ChemComp-MAN: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.8 µm Bright-field microscopy / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.8 µm |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Initial angle assignment | Type: OTHER / Software - Name: RELION (ver. 3.1) / Details: Initial Euler Angle Assignment |
| Final angle assignment | Type: OTHER / Software - Name: RELION (ver. 3.1) / Details: Final Euler Angle Assignment |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.0 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 1265104 |
 Movie
Movie Controller
Controller







 Z
Z Y
Y X
X

























