+ Open data
Open data
- Basic information
Basic information
| Entry | 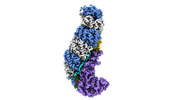 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
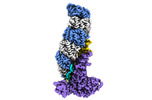
| Title | Structure of type I-D Cascade bound to a ssRNA target | |||||||||
 Map data Map data | CRISPR-Cas type I-D complex bound to an ssRNA target | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  CRISPR / CRISPR /  Complex / Complex /  Ribonucleoprotein complex / type I-D / type ID / type I / Ribonucleoprotein complex / type I-D / type ID / type I /  cyanobacteria / cyanobacteria /  synechocystis / synechocystis /  RNA binding protein / RNA BINDING PROTEIN-RNA complex RNA binding protein / RNA BINDING PROTEIN-RNA complex | |||||||||
| Function / homology | CRISPR-associated protein Csc1 / CRISPR associated protein Csc3 / CRISPR-associated protein Csc2 / Csc2 Crispr / Slr7013 protein / Slr7012 protein / CRISPR-associated protein Csc3 Function and homology information Function and homology information | |||||||||
| Biological species |   Synechocystis sp. PCC 6803 (bacteria) / synthetic construct (others) Synechocystis sp. PCC 6803 (bacteria) / synthetic construct (others) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.1 Å cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Schwartz EA / Taylor DW | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Structural rearrangements allow nucleic acid discrimination by type I-D Cascade. Authors: Evan A Schwartz / Tess M McBride / Jack P K Bravo / Daniel Wrapp / Peter C Fineran / Robert D Fagerlund / David W Taylor /   Abstract: CRISPR-Cas systems are adaptive immune systems that protect prokaryotes from foreign nucleic acids, such as bacteriophages. Two of the most prevalent CRISPR-Cas systems include type I and type III. ...CRISPR-Cas systems are adaptive immune systems that protect prokaryotes from foreign nucleic acids, such as bacteriophages. Two of the most prevalent CRISPR-Cas systems include type I and type III. Interestingly, the type I-D interference proteins contain characteristic features of both type I and type III systems. Here, we present the structures of type I-D Cascade bound to both a double-stranded (ds)DNA and a single-stranded (ss)RNA target at 2.9 and 3.1 Å, respectively. We show that type I-D Cascade is capable of specifically binding ssRNA and reveal how PAM recognition of dsDNA targets initiates long-range structural rearrangements that likely primes Cas10d for Cas3' binding and subsequent non-target strand DNA cleavage. These structures allow us to model how binding of the anti-CRISPR protein AcrID1 likely blocks target dsDNA binding via competitive inhibition of the DNA substrate engagement with the Cas10d active site. This work elucidates the unique mechanisms used by type I-D Cascade for discrimination of single-stranded and double stranded targets. Thus, our data supports a model for the hybrid nature of this complex with features of type III and type I systems. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24976.map.gz emd_24976.map.gz | 290.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24976-v30.xml emd-24976-v30.xml emd-24976.xml emd-24976.xml | 18.7 KB 18.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_24976_fsc.xml emd_24976_fsc.xml | 15.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_24976.png emd_24976.png | 39.7 KB | ||
| Filedesc metadata |  emd-24976.cif.gz emd-24976.cif.gz | 7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24976 http://ftp.pdbj.org/pub/emdb/structures/EMD-24976 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24976 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24976 | HTTPS FTP |
-Related structure data
| Related structure data |  7sbbMC  7sbaC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_24976.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24976.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CRISPR-Cas type I-D complex bound to an ssRNA target | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.045 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Type I-D Cascade bound to a ssRNA target
| Entire | Name: Type I-D Cascade bound to a ssRNA target |
|---|---|
| Components |
|
-Supramolecule #1: Type I-D Cascade bound to a ssRNA target
| Supramolecule | Name: Type I-D Cascade bound to a ssRNA target / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Molecular weight | Theoretical: 450 KDa |
-Macromolecule #1: Cas7d
| Macromolecule | Name: Cas7d / type: protein_or_peptide / ID: 1 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Synechocystis sp. PCC 6803 (bacteria) Synechocystis sp. PCC 6803 (bacteria) |
| Molecular weight | Theoretical: 36.527109 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MLDSLKSQFQ PSFPRLASGH YVHFLMLRHS QSFPVFQTDG VLNTTRTQAG LLEKTDQLSR LVMFKRKQTT PERLAGRELL RNLGLTSAD KSAKNLCEYN GEGSCKQCPD CILYGFAIGD SGSERSKVYS DSAFSLGAYE QSHRSFTFNA PFEGGTMSEA G VMRSAINE ...String: MLDSLKSQFQ PSFPRLASGH YVHFLMLRHS QSFPVFQTDG VLNTTRTQAG LLEKTDQLSR LVMFKRKQTT PERLAGRELL RNLGLTSAD KSAKNLCEYN GEGSCKQCPD CILYGFAIGD SGSERSKVYS DSAFSLGAYE QSHRSFTFNA PFEGGTMSEA G VMRSAINE LDHILPEVTF PTVESLRDAT YEGFIYVLGN LLRTKRYGAQ ESRTGTMKNH LVGIVFADGE IFSNLHLTQA LY DQMGGEL NKPISELCET AATVAQDLLN KEPVRKSELI FGAHLDTLLQ EVNDIYQNDA ELTKLLGSLY QQTQDYATEF GAL SGGKKK AKS UniProtKB: Slr7012 protein |
-Macromolecule #2: Cas5d
| Macromolecule | Name: Cas5d / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Synechocystis sp. PCC 6803 (bacteria) Synechocystis sp. PCC 6803 (bacteria) |
| Molecular weight | Theoretical: 28.942748 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MTKIYRCKLT LHDNVFFASR EMGILYETEK YFHNWALSYA FFKGTIIPHP YGLVGQNAQT PAYLDRDREQ NLLHLNDSGI YVFPAQPIH WSYQINTFKA AQSAYYGRSV QFGGKGATKN YPINYGRAKE LAVGSEFLTY IVSQKELDLP VWIRLGKWSS K IRVEVEAI ...String: MTKIYRCKLT LHDNVFFASR EMGILYETEK YFHNWALSYA FFKGTIIPHP YGLVGQNAQT PAYLDRDREQ NLLHLNDSGI YVFPAQPIH WSYQINTFKA AQSAYYGRSV QFGGKGATKN YPINYGRAKE LAVGSEFLTY IVSQKELDLP VWIRLGKWSS K IRVEVEAI APDQIKTASG VYVCNHPLNP LDCPANQQIL LYNRVVMPPS SLFSQSQLQG DYWQIDRNTF LPQGFHYGAT TA IAQDSPQ LSLLDTN UniProtKB: Slr7013 protein |
-Macromolecule #3: Cas10d
| Macromolecule | Name: Cas10d / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Synechocystis sp. PCC 6803 (bacteria) Synechocystis sp. PCC 6803 (bacteria) |
| Molecular weight | Theoretical: 112.067508 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MTTLLQTLLI RTLSEQKDYI LLEYFQTILP ALEEHFGNTS GLGGSFISHQ KHFGTQGYDT EKAKKMAQGF AKKGDQTLAA HILNALLTT WNVMQELEFP LNDIERRLLC LGITLHDYDK HCHAQDMAAP EPDNIQEIIN ICLELGKRLN FDEFWADWRD Y IAEISYLA ...String: MTTLLQTLLI RTLSEQKDYI LLEYFQTILP ALEEHFGNTS GLGGSFISHQ KHFGTQGYDT EKAKKMAQGF AKKGDQTLAA HILNALLTT WNVMQELEFP LNDIERRLLC LGITLHDYDK HCHAQDMAAP EPDNIQEIIN ICLELGKRLN FDEFWADWRD Y IAEISYLA QNTHGKQHTN LISSNWSNAG YPFTIKERKL DHPLRHLLTF GDVAVHLSSP HDLVSSTMGD RLRDLLNRLG IE KRFVYHH LRDTTGILSN AIHNVILRTV QKLDWKPLLF FAQGVIYFAP QDTEIPERNE IKQIVWQGIS QELGKKMSAG DVG FKRDGK GLKVSPQTSE LLAAADIVRI LPQVISVKVN NAKSPATPKR LEKLELGDAE REKLYEVADL RCDRLAELLG LVQK EIFLL PEPFIEWVLK DLELTSVIMP EETQVQSGGV NYGWYRVAAH YVANHATWDL EEFQEFLQGF GDRLATWAEE EGYFA EHQS PTRQIFEDYL DRYLEIQGWE SDHQAFIQEL ENYVNAKTKK SKQPICSLSS GEFPSEDQMD SVVLFKPQQY SNKNPL GGG QIKRGISKIW SLEMLLRQAF WSVPSGKFED QQPIFIYLYP AYVYAPQVVE AIRELVYGIA SVNLWDVRKH WVNNKMD LT SLKSLPWLNE EVEAGTNAQL KYTKEDLPFL ATVYTTTREK TDTDAWVKPA FLALLLPYLL GVKAIATRSM VPLYRSDQ D FRESIHLDGV AGFWSLLGIP TDLRVEDITP ALNKLLAIYT LHLAARSSPP KARWQDLPKT VQEVMTDVLN VFALAEQGL RREKRDRPYE SEVTEYWQFA ELFSQGNIVM TEKLKLTKRL VEEYRRFYQV ELSKKPSTHA ILLPLSKALE QILSVPDDWD EEELILQGS GQLQAALDRQ EVYTRPIIKD KSVAYETRQL QELEAIQIFM TTCVRDLFGE MCKGDRAILQ EQRNRIKSGA E FAYRLLAL EAQQNQN UniProtKB: CRISPR-associated protein Csc3 |
-Macromolecule #4: Cas11d
| Macromolecule | Name: Cas11d / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Synechocystis sp. PCC 6803 (bacteria) Synechocystis sp. PCC 6803 (bacteria) |
| Molecular weight | Theoretical: 17.024494 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MTEKLKLTKR LVEEYRRFYQ VELSKKPSTH AILLPLSKAL EQILSVPDDW DEEELILQGS GQLQAALDRQ EVYTRPIIKD KSVAYETRQ LQELEAIQIF MTTCVRDLFG EMCKGDRAIL QEQRNRIKSG AEFAYRLLAL EAQQNQN UniProtKB: CRISPR-associated protein Csc3 |
-Macromolecule #5: ssRNA target
| Macromolecule | Name: ssRNA target / type: rna / ID: 5 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 10.700537 KDa |
| Sequence | String: AGGCAUUGAA AGCGACCACC AGGGGCACAA CAA |
-Macromolecule #6: crRNA
| Macromolecule | Name: crRNA / type: rna / ID: 6 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:   Synechocystis sp. PCC 6803 (bacteria) Synechocystis sp. PCC 6803 (bacteria) |
| Molecular weight | Theoretical: 13.699081 KDa |
| Sequence | String: ACUGAAACGA UUGUUGUGCC CCUGGCGGUC GCUUUCAAUG CCU GENBANK: GENBANK: CP073020.1 |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.16 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| |||||||||||||||
| Grid | Model: C-flat / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 30 sec. | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV Details: Blot force 0, blot time 4s, no wait time between sample application and blotting.. | |||||||||||||||
| Details | Monodisperse sample of elongated particles. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm / Nominal magnification: 22500 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm / Nominal magnification: 22500 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 2 / Average exposure time: 3.0 sec. / Average electron dose: 41.2 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Details | Model was built de novo using coot then flexibly refined using ISOLDE. |
|---|---|
| Output model |  PDB-7sbb: |
 Movie
Movie Controller
Controller