[English] 日本語
 Yorodumi
Yorodumi- EMDB-20284: Cryo-EM structure of Urocortin 1-bound Corticotropin-releasing fa... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20284 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Urocortin 1-bound Corticotropin-releasing factor 1 receptor in complex with Gs protein and Nb35 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of adenylate cyclase activity involved in G protein-coupled receptor signaling pathway /  corticotropin-releasing hormone binding / corticotropin-releasing hormone binding /  histone deacetylase inhibitor activity / regulation of corticosterone secretion / corticotrophin-releasing factor receptor activity / histone deacetylase inhibitor activity / regulation of corticosterone secretion / corticotrophin-releasing factor receptor activity /  corticotropin-releasing hormone receptor 2 binding / corticotropin secretion / corticotropin-releasing hormone receptor 2 binding / corticotropin secretion /  general adaptation syndrome, behavioral process / positive regulation of corticotropin secretion / cellular response to corticotropin-releasing hormone stimulus ...regulation of adenylate cyclase activity involved in G protein-coupled receptor signaling pathway / general adaptation syndrome, behavioral process / positive regulation of corticotropin secretion / cellular response to corticotropin-releasing hormone stimulus ...regulation of adenylate cyclase activity involved in G protein-coupled receptor signaling pathway /  corticotropin-releasing hormone binding / corticotropin-releasing hormone binding /  histone deacetylase inhibitor activity / regulation of corticosterone secretion / corticotrophin-releasing factor receptor activity / histone deacetylase inhibitor activity / regulation of corticosterone secretion / corticotrophin-releasing factor receptor activity /  corticotropin-releasing hormone receptor 2 binding / corticotropin secretion / corticotropin-releasing hormone receptor 2 binding / corticotropin secretion /  general adaptation syndrome, behavioral process / positive regulation of corticotropin secretion / cellular response to corticotropin-releasing hormone stimulus / positive regulation of behavioral fear response / general adaptation syndrome, behavioral process / positive regulation of corticotropin secretion / cellular response to corticotropin-releasing hormone stimulus / positive regulation of behavioral fear response /  parturition / negative regulation of hormone secretion / negative regulation of voltage-gated calcium channel activity / varicosity / response to auditory stimulus / parturition / negative regulation of hormone secretion / negative regulation of voltage-gated calcium channel activity / varicosity / response to auditory stimulus /  drinking behavior / behavioral response to ethanol / negative regulation of appetite / drinking behavior / behavioral response to ethanol / negative regulation of appetite /  corticotropin-releasing hormone receptor 1 binding / neuropeptide hormone activity / fear response / G protein-coupled peptide receptor activity / positive regulation of vascular permeability / negative regulation of cell size / Class B/2 (Secretin family receptors) / exploration behavior / response to pain / adrenal gland development / negative regulation of feeding behavior / corticotropin-releasing hormone receptor 1 binding / neuropeptide hormone activity / fear response / G protein-coupled peptide receptor activity / positive regulation of vascular permeability / negative regulation of cell size / Class B/2 (Secretin family receptors) / exploration behavior / response to pain / adrenal gland development / negative regulation of feeding behavior /  startle response / positive regulation of calcium ion import / startle response / positive regulation of calcium ion import /  social behavior / PKA activation in glucagon signalling / social behavior / PKA activation in glucagon signalling /  associative learning / hair follicle placode formation / developmental growth / associative learning / hair follicle placode formation / developmental growth /  D1 dopamine receptor binding / neuropeptide signaling pathway / Adenylate cyclase inhibitory pathway / positive regulation of collagen biosynthetic process / positive regulation of protein localization to cell cortex / D1 dopamine receptor binding / neuropeptide signaling pathway / Adenylate cyclase inhibitory pathway / positive regulation of collagen biosynthetic process / positive regulation of protein localization to cell cortex /  intracellular transport / Synthesis, secretion, and deacylation of Ghrelin / regulation of cAMP-mediated signaling / D2 dopamine receptor binding / G protein-coupled serotonin receptor binding / Hedgehog 'off' state / positive regulation of cAMP-mediated signaling / axon terminus / intracellular transport / Synthesis, secretion, and deacylation of Ghrelin / regulation of cAMP-mediated signaling / D2 dopamine receptor binding / G protein-coupled serotonin receptor binding / Hedgehog 'off' state / positive regulation of cAMP-mediated signaling / axon terminus /  aerobic respiration / adenylate cyclase-activating adrenergic receptor signaling pathway / regulation of mitotic spindle organization / response to glucocorticoid / cellular response to forskolin / aerobic respiration / adenylate cyclase-activating adrenergic receptor signaling pathway / regulation of mitotic spindle organization / response to glucocorticoid / cellular response to forskolin /  regulation of synaptic transmission, glutamatergic / positive regulation of cardiac muscle contraction / activation of adenylate cyclase activity / negative regulation of blood pressure / adenylate cyclase activator activity / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / positive regulation of translation / trans-Golgi network membrane / positive regulation of DNA replication / female pregnancy / Regulation of insulin secretion / G protein-coupled receptor binding / sensory perception of sound / Olfactory Signaling Pathway / G-protein beta/gamma-subunit complex binding / Activation of the phototransduction cascade / regulation of synaptic transmission, glutamatergic / positive regulation of cardiac muscle contraction / activation of adenylate cyclase activity / negative regulation of blood pressure / adenylate cyclase activator activity / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / positive regulation of translation / trans-Golgi network membrane / positive regulation of DNA replication / female pregnancy / Regulation of insulin secretion / G protein-coupled receptor binding / sensory perception of sound / Olfactory Signaling Pathway / G-protein beta/gamma-subunit complex binding / Activation of the phototransduction cascade /  bone development / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / G-protein activation / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / ADP signalling through P2Y purinoceptor 12 / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / Sensory perception of sweet, bitter, and umami (glutamate) taste / bone development / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / G-protein activation / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / ADP signalling through P2Y purinoceptor 12 / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / Sensory perception of sweet, bitter, and umami (glutamate) taste /  platelet aggregation / response to peptide hormone / photoreceptor disc membrane / platelet aggregation / response to peptide hormone / photoreceptor disc membrane /  cognition / Adrenaline,noradrenaline inhibits insulin secretion / Glucagon-type ligand receptors / Vasopressin regulates renal water homeostasis via Aquaporins / positive regulation of GTPase activity / G alpha (z) signalling events / cellular response to catecholamine stimulus / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / ADORA2B mediated anti-inflammatory cytokines production cognition / Adrenaline,noradrenaline inhibits insulin secretion / Glucagon-type ligand receptors / Vasopressin regulates renal water homeostasis via Aquaporins / positive regulation of GTPase activity / G alpha (z) signalling events / cellular response to catecholamine stimulus / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / ADORA2B mediated anti-inflammatory cytokines productionSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) / synthetic construct (others) Homo sapiens (human) / synthetic construct (others) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.0 Å cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Ma S / Shen Q / Zhao L-H / Mao C / Zhou XE / Shen D-D / de Waal PW / Bi P / Li C / Jiang Y ...Ma S / Shen Q / Zhao L-H / Mao C / Zhou XE / Shen D-D / de Waal PW / Bi P / Li C / Jiang Y / Wang M-W / Sexton PM / Wootten D / Melcher K / Zhang Y / Xu HE | |||||||||
| Funding support |  United States, United States,  China, 2 items China, 2 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2020 Journal: Mol Cell / Year: 2020Title: Molecular Basis for Hormone Recognition and Activation of Corticotropin-Releasing Factor Receptors. Authors: Shanshan Ma / Qingya Shen / Li-Hua Zhao / Chunyou Mao / X Edward Zhou / Dan-Dan Shen / Parker W de Waal / Peng Bi / Chuntao Li / Yi Jiang / Ming-Wei Wang / Patrick M Sexton / Denise Wootten ...Authors: Shanshan Ma / Qingya Shen / Li-Hua Zhao / Chunyou Mao / X Edward Zhou / Dan-Dan Shen / Parker W de Waal / Peng Bi / Chuntao Li / Yi Jiang / Ming-Wei Wang / Patrick M Sexton / Denise Wootten / Karsten Melcher / Yan Zhang / H Eric Xu /    Abstract: Corticotropin-releasing factor (CRF) and the three related peptides urocortins 1-3 (UCN1-UCN3) are endocrine hormones that control the stress responses by activating CRF1R and CRF2R, two members of ...Corticotropin-releasing factor (CRF) and the three related peptides urocortins 1-3 (UCN1-UCN3) are endocrine hormones that control the stress responses by activating CRF1R and CRF2R, two members of class B G-protein-coupled receptors (GPCRs). Here, we present two cryoelectron microscopy (cryo-EM) structures of UCN1-bound CRF1R and CRF2R with the stimulatory G protein. In both structures, UCN1 adopts a single straight helix with its N terminus dipped into the receptor transmembrane bundle. Although the peptide-binding residues in CRF1R and CRF2R are different from other members of class B GPCRs, the residues involved in receptor activation and G protein coupling are conserved. In addition, both structures reveal bound cholesterol molecules to the receptor transmembrane helices. Our structures define the basis of ligand-binding specificity in the CRF receptor-hormone system, establish a common mechanism of class B GPCR activation and G protein coupling, and provide a paradigm for studying membrane protein-lipid interactions for class B GPCRs. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20284.map.gz emd_20284.map.gz | 35.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20284-v30.xml emd-20284-v30.xml emd-20284.xml emd-20284.xml | 17.4 KB 17.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_20284.png emd_20284.png | 40.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20284 http://ftp.pdbj.org/pub/emdb/structures/EMD-20284 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20284 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20284 | HTTPS FTP |
-Related structure data
| Related structure data |  6pb0MC  6pb1C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20284.map.gz / Format: CCP4 / Size: 38.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20284.map.gz / Format: CCP4 / Size: 38.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.014 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Urocortin1-bound CRF1R in complex Gs and Nb35
| Entire | Name: Urocortin1-bound CRF1R in complex Gs and Nb35 |
|---|---|
| Components |
|
-Supramolecule #1: Urocortin1-bound CRF1R in complex Gs and Nb35
| Supramolecule | Name: Urocortin1-bound CRF1R in complex Gs and Nb35 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#6 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
-Macromolecule #1: Corticotropin-releasing factor receptor 1
| Macromolecule | Name: Corticotropin-releasing factor receptor 1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 43.509711 KDa |
| Recombinant expression | Organism:  Spodoptera aff. frugiperda 2 RZ-2014 (butterflies/moths) Spodoptera aff. frugiperda 2 RZ-2014 (butterflies/moths) |
| Sequence | String: SLQDQHCESL SLASNISGLQ CNASVDLIGT CWPRSPAGQL VVRPCPAFFY GVRYNTTNNG YRECLANGSW AARVNYSECQ EILNEEKKS KVHYHVAVII NYLGHCISLV ALLVAFVLFL RLRSIRCLRN IIHWNLISAF ILRNATWFVV QLTMSPEVHQ S NVGWCRLV ...String: SLQDQHCESL SLASNISGLQ CNASVDLIGT CWPRSPAGQL VVRPCPAFFY GVRYNTTNNG YRECLANGSW AARVNYSECQ EILNEEKKS KVHYHVAVII NYLGHCISLV ALLVAFVLFL RLRSIRCLRN IIHWNLISAF ILRNATWFVV QLTMSPEVHQ S NVGWCRLV TAAYNYFHVT NFFWMFGEGC YLHTAIVLTY STDRLRKWMF ICIGWGVPFP IIVAWAIGKL YYDNEKCWFG KR PGVYTDY IYQGPMILVL LINFIFLFNI VRILMTKLRA STTSETIQYR KAVKATLVLL PLLGITYMLF FVNPGEDEVS RVV FIYFNS FLESFQGFFV SVFYCFLNSE VRSAIRKRWH RWQDKHSIRA RVARAMSIP |
-Macromolecule #2: Urocortin
| Macromolecule | Name: Urocortin / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 4.703277 KDa |
| Recombinant expression | Organism: synthetic construct (others) |
| Sequence | String: DNPSLSIDLT FHLLRTLLEL ARTQSQRERA EQNRIIFDSV |
-Macromolecule #3: Guanine nucleotide-binding protein G(s) subunit alpha isoforms sh...
| Macromolecule | Name: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short,Guanine nucleotide-binding protein G(i) subunit alpha-1,Guanine nucleotide-binding protein G(s) subunit alpha isoforms short type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 43.897789 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MGCLGNSKTE DQRNEEKAQR EANKKIEKQL QKDKQVYRAT HRLLLLGAGE SGKSTIVKQM RILHVNGYSE EECKQYKAVV YSNTIQSII AIIRAMGRLK IDFGDSARAD DARQLFVLAG AAEEGFMTAE LAGVIKRLWK DSGVQACFNR SREYQLNDSA A YYLNDLDR ...String: MGCLGNSKTE DQRNEEKAQR EANKKIEKQL QKDKQVYRAT HRLLLLGAGE SGKSTIVKQM RILHVNGYSE EECKQYKAVV YSNTIQSII AIIRAMGRLK IDFGDSARAD DARQLFVLAG AAEEGFMTAE LAGVIKRLWK DSGVQACFNR SREYQLNDSA A YYLNDLDR IAQPNYIPTQ QDVLRTRVKT TGIFETKFQV DKVNFHMFDV GAQRDERRKW IQCFNDVTAI IFVVASSSYN MV IREDNQT NRLQEALNLF KSIWNNRWLR TISVILFLNK QDLLAEKVLA GKSKIEDYFP EFARYTTPED ATPEPGEDPR VTR AKYFIR DEFLRISTAS GDGRHYCYPH FTCSVDTENI RRVFNDCRDI IQRMHLRQYE LL |
-Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.915496 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MGSLLQSELD QLRQEAEQLK NQIRDARKAC ADATLSQITN NIDPVGRIQM RTRRTLRGHL AKIYAMHWGT DSRLLVSASQ DGKLIIWDS YTTNKVHAIP LRSSWVMTCA YAPSGNYVAC GGLDNICSIY NLKTREGNVR VSRELAGHTG YLSCCRFLDD N QIVTSSGD ...String: MGSLLQSELD QLRQEAEQLK NQIRDARKAC ADATLSQITN NIDPVGRIQM RTRRTLRGHL AKIYAMHWGT DSRLLVSASQ DGKLIIWDS YTTNKVHAIP LRSSWVMTCA YAPSGNYVAC GGLDNICSIY NLKTREGNVR VSRELAGHTG YLSCCRFLDD N QIVTSSGD TTCALWDIET GQQTTTFTGH TGDVMSLSLA PDTRLFVSGA CDASAKLWDV REGMCRQTFT GHESDINAIC FF PNGNAFA TGSDDATCRL FDLRADQELM TYSHDNIICG ITSVSFSKSG RLLLAGYDDF NCNVWDALKA DRAGVLAGHD NRV SCLGVT DDGMAVATGS WDSFLKIWN |
-Macromolecule #5: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L |
-Macromolecule #6: Nanobody 35
| Macromolecule | Name: Nanobody 35 / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 15.343019 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MAQVQLQESG GGLVQPGGSL RLSCAASGFT FSNYKMNWVR QAPGKGLEWV SDISQSGASI SYTGSVKGRF TISRDNAKNT LYLQMNSLK PEDTAVYYCA RCPAPFTRDC FDVTSTTYAY RGQGTQVTVS SHHHHHHEPE A |
-Macromolecule #7: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 7 / Number of copies: 5 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information | 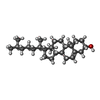 ChemComp-CLR: |
-Macromolecule #8: PALMITIC ACID
| Macromolecule | Name: PALMITIC ACID / type: ligand / ID: 8 / Number of copies: 7 / Formula: PLM |
|---|---|
| Molecular weight | Theoretical: 256.424 Da |
| Chemical component information |  ChemComp-PLM: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Details: unspecified |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy Bright-field microscopy |
| Image recording | Film or detector model: GATAN K2 BASE (4k x 4k) / Average electron dose: 66.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Initial angle assignment | Type: NOT APPLICABLE |
|---|---|
| Final angle assignment | Type: NOT APPLICABLE |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.0 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 97386 |
 Movie
Movie Controller
Controller






































