+Search query
-Structure paper
| Title | Conserved structural elements specialize ATAD1 as a membrane protein extraction machine. |
|---|---|
| Journal, issue, pages | Elife, Vol. 11, Year 2022 |
| Publish date | May 12, 2022 |
 Authors Authors | Lan Wang / Hannah Toutkoushian / Vladislav Belyy / Claire Y Kokontis / Peter Walter /  |
| PubMed Abstract | The mitochondrial AAA (TPase ssociated with diverse cellular ctivities) protein ATAD1 (in humans; Msp1 in yeast) removes mislocalized membrane proteins, as well as stuck import substrates from the ...The mitochondrial AAA (TPase ssociated with diverse cellular ctivities) protein ATAD1 (in humans; Msp1 in yeast) removes mislocalized membrane proteins, as well as stuck import substrates from the mitochondrial outer membrane, facilitating their re-insertion into their cognate organelles and maintaining mitochondria's protein import capacity. In doing so, it helps to maintain proteostasis in mitochondria. How ATAD1 tackles the energetic challenge to extract hydrophobic membrane proteins from the lipid bilayer and what structural features adapt ATAD1 for its particular function has remained a mystery. Previously, we determined the structure of Msp1 in complex with a peptide substrate (Wang et al., 2020). The structure showed that Msp1's mechanism follows the general principle established for AAA proteins while adopting several structural features that specialize it for its function. Among these features in Msp1 was the utilization of multiple aromatic amino acids to firmly grip the substrate in the central pore. However, it was not clear whether the aromatic nature of these amino acids were required, or if they could be functionally replaced by aliphatic amino acids. In this work, we determined the cryo-EM structures of the human ATAD1 in complex with a peptide substrate at near atomic resolution. The structures show that phylogenetically conserved structural elements adapt ATAD1 for its function while generally adopting a conserved mechanism shared by many AAA proteins. We developed a microscopy-based assay reporting on protein mislocalization, with which we directly assessed ATAD1's activity in live cells and showed that both aromatic amino acids in pore-loop 1 are required for ATAD1's function and cannot be substituted by aliphatic amino acids. A short α-helix at the C-terminus strongly facilitates ATAD1's oligomerization, a structural feature that distinguishes ATAD1 from its closely related proteins. |
 External links External links |  Elife / Elife /  PubMed:35550246 / PubMed:35550246 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 3.2 - 3.5 Å |
| Structure data | EMDB-26674, PDB-7upr: EMDB-26675, PDB-7upt: |
| Chemicals | 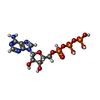 ChemComp-ATP:  ChemComp-MG: 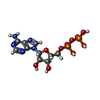 ChemComp-ADP: |
| Source |
|
 Keywords Keywords |  PROTEIN TRANSPORT / PROTEIN TRANSPORT /  AAA protein / AAA protein /  mitochondria / tail-anchored protein / mitochondria / tail-anchored protein /  membrane protein membrane protein |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers









