+Search query
-Structure paper
| Title | Parental histone transfer caught at the replication fork. |
|---|---|
| Journal, issue, pages | Nature, Vol. 627, Issue 8005, Page 890-897, Year 2024 |
| Publish date | Mar 6, 2024 |
 Authors Authors | Ningning Li / Yuan Gao / Yujie Zhang / Daqi Yu / Jianwei Lin / Jianxun Feng / Jian Li / Zhichun Xu / Yingyi Zhang / Shangyu Dang / Keda Zhou / Yang Liu / Xiang David Li / Bik Kwoon Tye / Qing Li / Ning Gao / Yuanliang Zhai /   |
| PubMed Abstract | In eukaryotes, DNA compacts into chromatin through nucleosomes. Replication of the eukaryotic genome must be coupled to the transmission of the epigenome encoded in the chromatin. Here we report cryo- ...In eukaryotes, DNA compacts into chromatin through nucleosomes. Replication of the eukaryotic genome must be coupled to the transmission of the epigenome encoded in the chromatin. Here we report cryo-electron microscopy structures of yeast (Saccharomyces cerevisiae) replisomes associated with the FACT (facilitates chromatin transactions) complex (comprising Spt16 and Pob3) and an evicted histone hexamer. In these structures, FACT is positioned at the front end of the replisome by engaging with the parental DNA duplex to capture the histones through the middle domain and the acidic carboxyl-terminal domain of Spt16. The H2A-H2B dimer chaperoned by the carboxyl-terminal domain of Spt16 is stably tethered to the H3-H4 tetramer, while the vacant H2A-H2B site is occupied by the histone-binding domain of Mcm2. The Mcm2 histone-binding domain wraps around the DNA-binding surface of one H3-H4 dimer and extends across the tetramerization interface of the H3-H4 tetramer to the binding site of Spt16 middle domain before becoming disordered. This arrangement leaves the remaining DNA-binding surface of the other H3-H4 dimer exposed to additional interactions for further processing. The Mcm2 histone-binding domain and its downstream linker region are nested on top of Tof1, relocating the parental histones to the replisome front for transfer to the newly synthesized lagging-strand DNA. Our findings offer crucial structural insights into the mechanism of replication-coupled histone recycling for maintaining epigenetic inheritance. |
 External links External links |  Nature / Nature /  PubMed:38448592 PubMed:38448592 |
| Methods | EM (single particle) |
| Resolution | 3.5 - 3.9 Å |
| Structure data |  EMDB-38313: Structure of yeast replisome associated with FACT and histone hexamer, the region of FACT-Histones optimized local map  EMDB-38314: Structure of yeast replisome associated with FACT and histone hexamer,Conformation-2  EMDB-38315: Structure of yeast replisome associated with FACT and histone hexamer, the region of polymerase epsilon optimized local map  EMDB-38316: Structure of yeast replisome associated with FACT and histone hexamer, Conformation-1 EMDB-38317, PDB-8xgc: |
| Chemicals |  ChemComp-ZN: 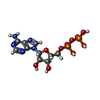 ChemComp-ADP: |
| Source |
|
 Keywords Keywords |  REPLICATION / REPLICATION /  Replisome / Replisome /  FACT / histone hexamer FACT / histone hexamer |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers






