+Search query
-Structure paper
| Title | Conformational plasticity of the ClpAP AAA+ protease couples protein unfolding and proteolysis. |
|---|---|
| Journal, issue, pages | Nat Struct Mol Biol, Vol. 27, Issue 5, Page 406-416, Year 2020 |
| Publish date | Apr 20, 2020 |
 Authors Authors | Kyle E Lopez / Alexandrea N Rizo / Eric Tse / JiaBei Lin / Nathaniel W Scull / Aye C Thwin / Aaron L Lucius / James Shorter / Daniel R Southworth /  |
| PubMed Abstract | The ClpAP complex is a conserved bacterial protease that unfolds and degrades proteins targeted for destruction. The ClpA double-ring hexamer powers substrate unfolding and translocation into the ...The ClpAP complex is a conserved bacterial protease that unfolds and degrades proteins targeted for destruction. The ClpA double-ring hexamer powers substrate unfolding and translocation into the ClpP proteolytic chamber. Here, we determined high-resolution structures of wild-type Escherichia coli ClpAP undergoing active substrate unfolding and proteolysis. A spiral of pore loop-substrate contacts spans both ClpA AAA+ domains. Protomers at the spiral seam undergo nucleotide-specific rearrangements, supporting substrate translocation. IGL loops extend flexibly to bind the planar, heptameric ClpP surface with the empty, symmetry-mismatched IGL pocket maintained at the seam. Three different structures identify a binding-pocket switch by the IGL loop of the lowest positioned protomer, involving release and re-engagement with the clockwise pocket. This switch is coupled to a ClpA rotation and a network of conformational changes across the seam, suggesting that ClpA can rotate around the ClpP apical surface during processive steps of translocation and proteolysis. |
 External links External links |  Nat Struct Mol Biol / Nat Struct Mol Biol /  PubMed:32313240 / PubMed:32313240 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.7 - 3.4 Å |
| Structure data | EMDB-20845, PDB-6uqe: EMDB-20851, PDB-6uqo: EMDB-21519, PDB-6w1z: EMDB-21520, PDB-6w20: EMDB-21521, PDB-6w21: EMDB-21522, PDB-6w22: EMDB-21523, PDB-6w23: EMDB-21524, PDB-6w24: |
| Chemicals |  ChemComp-ADP: 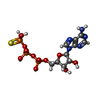 ChemComp-AGS: 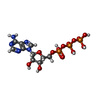 ChemComp-ATP: |
| Source |
|
 Keywords Keywords |  CHAPERONE / CHAPERONE /  AAA+ / AAA+ /  Protease / Protease /  Hsp100 / Hsp100 /  ATPase ATPase |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers




















